simple Powerpoint Templates Free Download 2007
(Last Updated: October 19, 2021)
For a successful PowerPoint presentation, rather than good content, it's necessary to mention the great contribution of an eye-catching visual design. The truth is that it helps keep your audience engaged from the first look, thus boosting your presentation. To create a professionally designed presentation, we need to spend much more time designing it. Honestly, a stunning ready-made template may help you get your work done in no time. To save your time and effort, you can search for free PowerPoint templates available on the Internet. If a wide variety of templates out there makes you overwhelmed, don't worry, this article is right for you. The list of top 10 websites helping you quickly find and download fantastic PowerPoint templates for free will be worth your consideration. Let's check it out.

Background psd created by jcomp – www.freepik.com
Top 10 websites for PowerPoint templates free download:
- Powerpointify
- Slidesgo
- Slides Carnival
- AllPPT.com
- Showeet
- Slide Hunter
- 24Slides
- Presentation Magazine
- fppt.com
- Powered Template
#1. Powerpointify
Created by top designers around the world, Powerpointify is truly a great place for high-quality PowerPoint templates for free download. You will be impressed with the professional design when visiting this website for the first time. That is to say, it hosts an amazingly wide range of free PowerPoint templates to present your presentations beautifully.
You can quickly search for templates by topics or through a search bar at the top of the page. With numerous categories, namely Business, Technology, Holiday, etc., these templates can meet the requirements of different projects.

Each template contains an interactive slideshow. There is also information about its color, design, layout, as well as its features. On account of that, you can get a better overview of that template before downloading it. One more cool thing is you can see ratings for a template by other users.
Pros
- The user interface is intuitive and easy to work with.
- Free templates are diverse and well-designed.
- It comes with no sign-up for free download.
- All elements in templates are fully editable in PowerPoint.
Cons
- It only supports downloading templates in ZIP file format, so you need to extract the files before using them.
#2. Slidesgo
When it comes to free PowerPoint templates, Slidesgo is one of the best providers. This website offers a lot of creative, colorful, and lovely templates for free download. Their delightful designs may help you capture your audience's attention from the first look. Templates are grouped into separated categories such as Education, Business, Marketing, Medical, etc. From here, you can quickly take a look at a topic you are interested in.
Apart from the free download option, the website also comes with the Premium package for you to choose from. This subscription plan gives you full access to endless resources of all Premium templates.
Pros
- It provides hundreds of eye-catching and high-quality PowerPoint templates for free download.
- You can freely search for templates by categories, colors, or tags.
- An interactive slideshow is available to see how slides look before you download them.
- Each download page contains helpful tutorials guiding you on the way to adjust templates to meet your needs.
- You can also download templates instantly without creating an account.
Cons
- It requires attribution in each freely downloaded template.
- As a Free user, if you want to download 10 free templates per month, you need to register on the website.
#3. Slides Carnival
Slides Carnival offers a great variety of professional PowerPoint template designs. By simply downloading a series of slides, you will reduce your designing process in minimal time. Templates provided on the Slides Carnival are all free for any use. Also, there are no registration or download limits. It's ideal for low-budget projects indeed. Take notice that, being of free resources, they are under the provider's Creative Commons Attribution license. However, you can normally do whatever you want. For example, changing colors, fonts, layouts, adding or deleting slides, etc. are at your fingertips.
The well-designed interface of the website has made its charm. That is to say, it clearly classifies a variety of topics for start-ups, businesses, or creative projects. Moreover, you can browse the most recent templates or filter them using the search bar at the top-right corner of the page. Each template will have about 35 – 40 slides with free attached icons and stickers. They are all freely customizable as you wish.
Pros
- PowerPoint templates are 100% free for both personal and commercial uses.
- You can easily search for templates on the site by topics, styles, colors, or type a keyword in the search bar.
- The website also provides explanatory information about each template and its features.
- It's quick and easy to download free templates with no registration.
Cons
- The slide design is quite simple.
#4. AllPPT.com
It would be an omission when not mentioned to AllPPT.com on the list. Let's say, it is an online resource of all PowerPoint stuff. This website gives you a chance to browse and download a fantastic collection of free PowerPoint templates. Not only that, photo graphics, diagrams, and charts are also ready to be used in your next presentations.
In general, AllPPT.com allows viewing templates quickly by organizing them into categories. In which, they are Education, Medical, Finance, Food, Travel, and many others. Browsing templates through categories or keywords may help speed up your work.

Pros
- Other than free PowerPoint templates, you can also download PowerPoint diagrams, illustrations, and free royalty background designs.
- Templates can be downloaded in .pptx file format for quick use.
- This website supports free templates that are compatible with Microsoft Powerpoint 2007, 2010, and 2013.
Cons
- You may be distracted by ads.
- It is fairly hard to find the Download button.
#5. Showeet
Showeet is another top website where you can download outstanding PowerPoint templates for free. Your business or educational presentations will be lightened up with colorful and attractive designs. Although this website doesn't categorize templates into groups, you can easily filter them by tags and keywords. Just click on popular keywords on the left side of the page, all the related templates are instantly shown for your choice.
Meanwhile, each download page shows a lot of information about usage instructions as well as a preview of slides. Taking a glance at this information may help you find whether that template is useful or meets your needs. Besides, before downloading a template, kindly check the Terms of Use carefully. This helps you make sure it's totally free and avoid any restrictions.
Pros
- Resume/Curriculum Vitae templates are available.
- Templates are updated and uploaded from time to time.
- You can download free templates immediately after hitting the Download button without any sign-up.
Cons
- You may take time to decompress the downloaded ZIP file for use.
#6. Slide Hunter
Though being an online free resource, Slide Hunter still provides you with dozens of engaging free PowerPoint templates. Using these templates will bring your presentations to the next level.
As you can see on the homepage, this website classifies templates into a wide range of categories. It's easy for you to find your preferred templates through categories as Business, Planning, Education, Charts, and much more. Besides, browsing templates through a search bar or popular keywords at the top of the page also comes in handy. Thanks to nice diagrams and graphics, some templates are ideal for making awesome business presentations. Also, unique shapes and 3D objects may help you impress your audience at a glance.
Pros
- Most templates have modern and creative designs.
- It has a detailed description of each template.
- You can instantly apply the downloaded templates which are in .pptx format to your presentations.
Cons
- The slides' preview is limited in some templates.
#7. 24Slides
This is another notable candidate in the race. Since 24Slides offers you a huge variety of free PowerPoint template choices. At the first look, you can see they are grouped by categories as other websites do. Agenda, Process, Marketing, Data are among those categories. By clicking on a template thumbnail, there are detailed explanations about it and a preview of slides. That then gives you a better understanding of the template uses. Thus, you can narrow down your choices.

Pros
- Templates are professionally and skillfully designed.
- You can also immediately use the downloaded templates that are stored in .pptx format.
Cons
- You need to create an account to download.
- It offers a shorter range of categories compared to other websites.
#8. Presentation Magazine
Presentation Magazine is a nice place for free PowerPoint templates as well. It offers +50,000 free and creative templates for you to choose from. The first impression when looking at this website is its simplicity. You can quickly find a template topic you need through a list of categories, for example, Animals, Calendar, Nature, etc. Not only that, browsing templates by popularity, colors or tags are also at your fingertips. After that, you can download your favorite ones by simply hitting the Download button without registration.
Pros
- The website offers both static and animated PowerPoint templates for free download.
- Templates are updated frequently.
Cons
- Its user interface is quite hard to work on. That is to say, categories are listed at the bottom of the page, so you need to scroll down to view them.
- The template's slide preview is limited.
- Most templates are with basic designs and include less than 10 slides for each.
#9. fppt.com
If you are looking for a website to freely download catchy PowerPoint templates, fppt.com is right for you. This website provides you with more than 2,000 stunning free templates.
Like other websites on the list, fppt.com also organizes its templates into various categories and tags, including Games, Maps, Nature, and so on. For your convenience, you can choose PowerPoint categories ordered alphabetically. That will help you narrow down your search process. Or if you prefer, you can also filter templates by keywords, themes, or backgrounds.
Pros
- Each download page displays a download count, so you can see its popularity from other users.
- No sign-up is required.
Cons
- Its categories aren't as rich compared to other websites on the list.
- It lacks the slide preview option.
- You have to wait for an ad before downloading.
- You need to extract the ZIP file after downloading a template.
- The number of slides in each template is limited.
#10. Powered Template
Last but not least, Powered Template is a worth-trying website for professional PowerPoint templates free download. Rather than templates for PowerPoint presentations, the website provides you with free ones for MS Word, CV, graphics, and more. At first glance, you can easily browse templates by types, categories, properties, ratios, and a number of slides on the left side of the page. Just tick any checkbox next to each option to filter your search. In each template thumbnail, it shows users' ratings and download counts, so you can consider your choice before downloading.
If you want to explore more fantastic templates this website brings out, you can try out the Premium subscription plan. From $19 per month, it's possible to gain full access, daily updates as well as timely support from the creators.
Pros
- Templates are designed with creative and eye-catching elements.
- Categories of PowerPoint templates are diverse to choose from.
Cons
- There is limited template's slide preview.
- You have to create an account to freely download.
- The downloaded ZIP file needs to be decompressed for use.
- Free templates are with attribution required.
Last Points
We've highlighted the top 10 websites letting you download PowerPoint templates for free. Hopefully, this article will help you find suitable templates, making your presentations gorgeous effortlessly.
For your further information, do you know that PowerPoint templates are not only used in MS PowerPoint? Let's say, with its great popularity, some eLearning authoring tools like ActivePresenter 8 allow importing PowerPoint templates into their platforms. Thanks to that, you can create much more customizable, interactive, and advanced eLearning courses.
As you may know, ActivePresenter 8 enables you to reuse PowerPoint files by importing them into the app for further editing. The significant possibility is you can do that easily even if MS PowerPoint isn't installed on your computer. After that, feel free to make your audience engaged better by converting those static slides into engagingly interactive eLearning courses. With the help of interactive objects, events – actions, variables, etc., your work will turn out to be easy as a piece of cake.
So, let's give ActivePresenter a try. And don't forget to visit our Blog and YouTube channel to get more useful eLearning content. Have a nice day!
Source: https://atomisystems.com/elearning/10-amazing-places-for-powerpoint-templates-free-download/
Posted by: hachigianroosevelt457.blogspot.com
powerpoint Presentation Slides 3d Design Free Download
Add a 3D animation to an opening or ending slides for your next PowerPoint presentation. It's easy to do, and it's sure to be a crowd-pleaser.

Image: Vladimka production/Shutterstock
Microsoft PowerPoint began distributing built-in 3D animations in 2018. There are a number of stock animations that you can freely include and distribute, and I think a perfect spot is the often boring beginning and ending slides. Most of the time, the slides you display when people are entering and finding a seat or leaving your presentation are a bit flat and dull. In this article, I'll show you how to add a bit of fun to these slides by inserting 3D animations. It's easy, but a lot of users don't know these new animations are available.
SEE: 83 Excel tips every user should master (TechRepublic)
I'm using Microsoft 365 on a Windows 10 64-system. This feature is available in recent versions of Microsoft 365, 2019 stand-alones, and Windows 10 phone apps. These animated objects are also available in Word, Excel and even Outlook. For your convenience, you can download the demonstration .pptx file. This feature isn't supported in the menu version.
What are they?
Microsoft refers to these animations as models, but I think 3D animation is just as meaningful and less confusing. It's not necessary to understand what happens out of sight. These 3D animations are a bit like morph only more fun and a lot less work. In fact, inserting a 3D animation is similar to inserting a gif. You insert the file, and it just works. Now, let's move on and insert a 3D animation into an opening slide.
SEE: Windows 10: Lists of vocal commands for speech recognition and dictation (free PDF) (TechRepublic)
How to insert a 3D animation into a PowerPoint slide
I've watched a lot of presentations, and while the opening and ending slides aren't even necessary, they do provide a hint to what's going to happen and when you're done. Most, sadly, have no sense of humor. Sometimes, that's appropriate, but 3D animations can provide a quick spark before and after your presentation.
Figure A shows a rather bland, but descriptive, slide that you might display as guests arrive to your workshop on multi-tasking. It's bland, but it does the job. Let's add a 3D animation to liven it up, just a bit.
Figure A

This opening slide introduces your topic, but it's boring.
To begin, you need an introductory slide. I used Comic Sans MS 66 for the title and 36 for the welcome line in the slide shown in Figure A. The text box is centered. Now, let's liven things up by inserting a 3D animation:
- Click the Insert tab.
- In the Illustrations group, click the 3D Models dropdown.
- From the dropdown, choose Stock 3D Models. Doing so opens an interface that lets you choose 3D animations by category.
- Click Animated Animals and browse down a bit until you find the juggling octopus shown in Figure B.
- Click Insert.
- Resize the 3D animation object until it's as large as it can be without extending off the slide.
- Move the text box up a bit so the animation doesn't obscure the text.
Figure B
Choose an animated octopus.
Figure C shows the slide in Normal view. Although you can't see the action in the figure, if you're following along, you can see that the octopus is juggling! Now that's a fun representation of multi-tasking.
Figure C
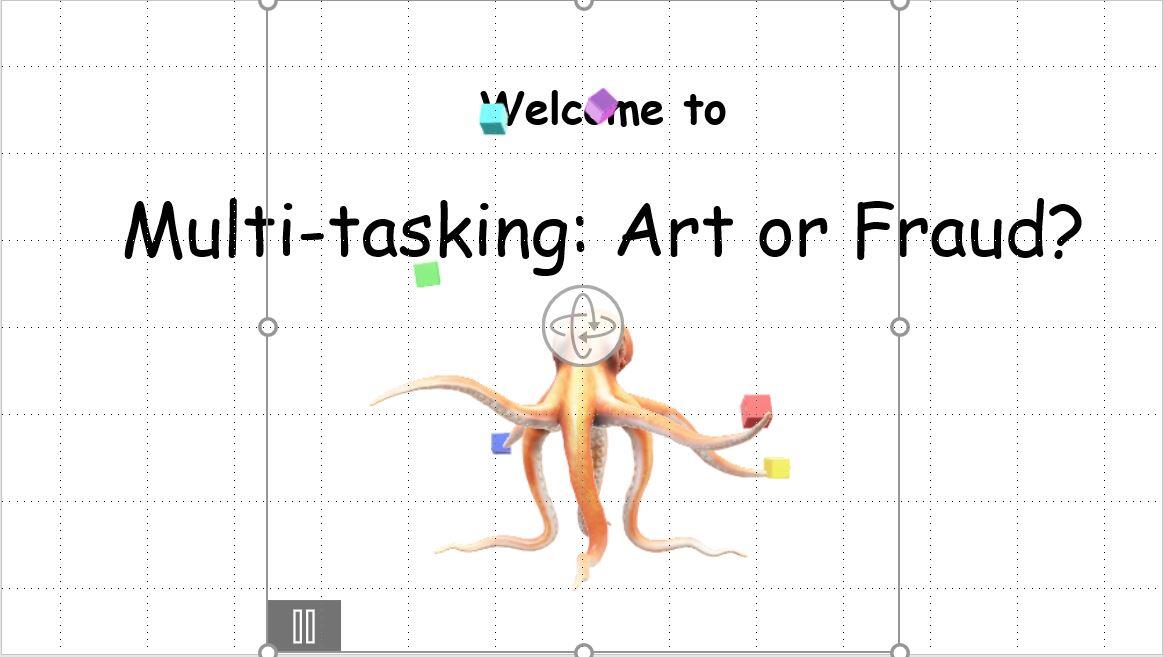
Add the 3D animation to the slide and resize and position as necessary.
Changing perspective and more
Did you notice the odd icon that looks a tiny bit like the atomic atom symbol in Figure C? This tool allows you to change the animation's perspective by rotating or tilting the view—remember, it's 3D. Figure D shows the result of dragging down and to the right just a bit. Because it's 3D, you see a completely different perspective—one from above instead of facing forward. Even the cubes change perspective with the octopus. If you don't like the changes, press Ctrl + Z to undo them.
Figure D

Change the perspective to see the different possibilities.
When the animation is selected, check out the contextual 3D Model ribbon shown in Figure E. These options offer even more ways to modify the animation to suit your needs. When you have a bit of time, explore these options further to get the most from your 3D animations.
Figure E
Use the contextual ribbon to customize the animation.
You'd expect to work much harder to achieve these effects. There's nothing difficult about adding a 3D animation, but the results can be awesome!

Microsoft Weekly Newsletter
Be your company's Microsoft insider by reading these Windows and Office tips, tricks, and cheat sheets. Delivered Mondays and Wednesdays
Sign up todayAlso see
- How to make fewer mistakes and work more efficiently using predictive text in Microsoft 365 (TechRepublic)
- How to use the many text wrapping options in Microsoft Word (TechRepublic)
- Microsoft 365: A cheat sheet (TechRepublic)
- Zoom vs. Microsoft Teams, Google Meet, Cisco WebEx and Skype: Choosing the right video-conferencing apps for you (free PDF) (TechRepublic)
- Checklist: Securing Windows 10 systems (TechRepublic Premium)
- Must-read coverage: Windows 10 (TechRepublic on Flipboard)
Posted by: hachigianroosevelt457.blogspot.com
Source: https://www.techrepublic.com/article/how-to-add-3d-animation-to-opening-and-ending-slides-in-powerpoint/
Ecg Goldberger Free Download Pdf
Table of Contents
Preamble e507
-
Introduction e508
1.1. Methodology and Evidence Review e508
1.2. Organization of the GWC e510
1.3. Document Review and Approval e510
1.4. Scope of the Guideline e510
-
General Principles e510
2.1. Mechanisms and Definitions e510
2.2. Epidemiology, Demographics, and Public Health Impact e510
2.3. Evaluation of the Patient With Suspected or Documented SVT e511
2.3.1. Clinical Presentation and Differential Diagnosis on the Basis of Symptoms e511
2.3.2. Evaluation of the ECG e514
2.4. Principles of Medical Therapy e515
2.4.1. Acute Treatment: Recommendations e515
2.4.2. Ongoing Management: Recommendations e517
2.5. Basic Principles of Electrophysiological Study, Mapping, and Ablation e518
2.5.1. Mapping With Multiple and Roving Electrodes e518
2.5.2. Tools to Facilitate Ablation, Including 3-Dimensional Electroanatomic Mapping e518
2.5.3. Mapping and Ablation With No or Minimal Radiation e519
2.5.4. Ablation Energy Sources e519
-
Sinus Tachyarrhythmias e520
3.1. Physiological Sinus Tachycardia e521
3.2. Inappropriate Sinus Tachycardia e521
3.2.1. Acute Treatment e521
3.2.2. Ongoing Management: Recommendations e521
-
Nonsinus Focal Atrial Tachycardia and MAT e523
4.1. Focal Atrial Tachycardia e523
4.1.1. Acute Treatment: Recommendations e526
4.1.2. Ongoing Management: Recommendations e527
4.2. Multifocal Atrial Tachycardia e527
4.2.1. Acute Treatment: Recommendation e531
4.2.2. Ongoing Management: Recommendations e531
-
Atrioventricular Nodal Reentrant Tachycardia e531
5.1. Acute Treatment: Recommendations e532
5.2. Ongoing Management: Recommendations e533
-
Manifest and Concealed Accessory Pathways e534
6.1. Management of Patients With Symptomatic Manifest or Concealed Accessory Pathways e535
6.1.1. Acute Treatment: Recommendations e535
6.1.2. Ongoing Management: Recommendations e536
6.2. Management of Asymptomatic Pre-Excitation e537
6.2.1. PICOTS Critical Questions e537
6.2.2. Asymptomatic Patients With Pre-Excitation: Recommendations e538
6.3. Risk Stratification of Symptomatic Patients With Manifest Accessory Pathways: Recommendations e539
-
Atrial Flutter e539
7.1. Cavotricuspid Isthmus–Dependent Atrial Flutter e539
7.2. Non–Isthmus-Dependent Atrial Flutters e540
7.3. Acute Treatment: Recommendations e541
7.4. Ongoing Management: Recommendations e542
-
Junctional Tachycardia e544
8.1. Acute Treatment: Recommendations e544
8.2. Ongoing Management: Recommendations e545
-
Special Populations e545
9.1. Pediatrics e545
9.2. Patients With Adult Congenital Heart Disease e549
9.2.1. Clinical Features e549
9.2.2. Acute Treatment: Recommendations e550
9.2.3. Ongoing Management: Recommendations e551
9.3. Pregnancy e553
9.3.1. Acute Treatment: Recommendations e553
9.3.2. Ongoing Management: Recommendations e554
9.4. SVT in Older Populations e555
9.4.1. Acute Treatment and Ongoing Management: Recommendation e555
-
Quality-of-Life Considerations e555
-
Cost-Effectiveness e555
-
Shared Decision Making e556
-
Evidence Gaps and Future Research Needs e556
| References e557 | |||||
| Appendix 1. Author Relationships With Industry and Other Entities (Relevant) e570 | |||||
| Appendix 2. Reviewer Relationships With Industry and Other Entities (Relevant) e571 | |||||
| Appendix 3. Abbreviations e574 | |||||
Preamble
Since 1980, the American College of Cardiology (ACC) and American Heart Association (AHA) have translated scientific evidence into clinical practice guidelines with recommendations to improve cardiovascular health. These guidelines, based on systematic methods to evaluate and classify evidence, provide a cornerstone of quality cardiovascular care.
In response to reports from the Institute of Medicine1,2 and a mandate to evaluate new knowledge and maintain relevance at the point of care, the ACC/AHA Task Force on Clinical Practice Guidelines (Task Force) modified its methodology.3–5 The relationships between guidelines, data standards, appropriate use criteria, and performance measures are addressed elsewhere.4
Intended Use
Practice guidelines provide recommendations applicable to patients with or at risk of developing cardiovascular disease. The focus is on medical practice in the United States, but guidelines developed in collaboration with other organizations may have a broader target. Although guidelines may inform regulatory or payer decisions, they are intended to improve quality of care in the interest of patients.
Evidence Review
Guideline Writing Committee (GWC) members review the literature; weigh the quality of evidence for or against particular tests, treatments, or procedures; and estimate expected health outcomes. In developing recommendations, the GWC uses evidence-based methodologies that are based on all available data.4–6 Literature searches focus on randomized controlled trials (RCTs) but also include registries, nonrandomized comparative and descriptive studies, case series, cohort studies, systematic reviews, and expert opinion. Only selected references are cited.
The Task Force recognizes the need for objective, independent Evidence Review Committees (ERCs) that include methodologists, epidemiologists, clinicians, and biostatisticians who systematically survey, abstract, and assess the evidence to address key clinical questions posed in the PICOTS format (P=population, I=intervention, C=comparator, O=outcome, T=timing, S=setting).4,5 Practical considerations, including time and resource constraints, limit the ERCs to evidence that is relevant to key clinical questions and lends itself to systematic review and analysis that could affect the strength of corresponding recommendations. Recommendations developed by the GWC on the basis of the systematic review are marked "SR".
Guideline-Directed Medical Therapy
The term guideline-directed medical therapy refers to care defined mainly by ACC/AHA Class I recommendations. For these and all recommended drug treatment regimens, the reader should confirm dosage with product insert material and carefully evaluate for contraindications and interactions. Recommendations are limited to treatments, drugs, and devices approved for clinical use in the United States.
Class of Recommendation and Level of Evidence
The Class of Recommendation (COR; ie, the strength of the recommendation) encompasses the anticipated magnitude and certainty of benefit in proportion to risk. The Level of Evidence (LOE) rates evidence supporting the effect of the intervention on the basis of the type, quality, quantity, and consistency of data from clinical trials and other reports (Table 1).5,7 Unless otherwise stated, recommendations are sequenced by COR and then by LOE. Where comparative data exist, preferred strategies take precedence. When >1 drug, strategy, or therapy exists within the same COR and LOE and no comparative data are available, options are listed alphabetically. Each recommendation is followed by supplemental text linked to supporting references and evidence tables.
 Table 1. Applying Class of Recommendation and Level of Evidence to Clinical Strategies, Interventions, Treatments, or Diagnostic Testing in Patient Care* |
Relationships With Industry and Other Entities
The ACC and AHA sponsor the guidelines without commercial support, and members volunteer their time. The Task Force zealously avoids actual, potential, or perceived conflicts of interest that might arise through relationships with industry or other entities (RWI). All GWC members and reviewers are required to disclose current industry relationships or personal interests from 12 months before initiation of the writing effort. Management of RWI involves selecting a balanced GWC and assuring that the chair and a majority of committee members have no relevant RWI (Appendix 1). Members are restricted with regard to writing or voting on sections to which their RWI apply. For transparency, members' comprehensive disclosure information is available online. Comprehensive disclosure information for the Task Force is also available online. The Task Force strives to avoid bias by selecting experts from a broad array of backgrounds representing different geographic regions, sexes, ethnicities, intellectual perspectives/biases, and scopes of clinical practice, and by inviting organizations and professional societies with related interests and expertise to participate as partners or collaborators.
Individualizing Care in Patients With Associated Conditions and Comorbidities
Managing patients with multiple conditions can be complex, especially when recommendations applicable to coexisting illnesses are discordant or interacting.8 The guidelines are intended to define practices meeting the needs of patients in most, but not all, circumstances. The recommendations should not replace clinical judgment.
Clinical Implementation
Management in accordance with guideline recommendations is effective only when followed. Adherence to recommendations can be enhanced by shared decision making between clinicians and patients, with patient engagement in selecting interventions based on individual values, preferences, and associated conditions and comorbidities. Consequently, circumstances may arise in which deviations from these guidelines are appropriate.
Policy
The recommendations in this guideline represent the official policy of the ACC and AHA until superseded by published addenda, statements of clarification, focused updates, or revised full-text guidelines. To ensure that guidelines remain current, new data are reviewed biannually to determine whether recommendations should be modified. In general, full revisions are posted in 5-year cycles.3,5
Jonathan L. Halperin, MD, FACC, FAHA
Chair, ACC/AHA Task Force on Clinical Practice Guidelines
1. Introduction
1.1. Methodology and Evidence Review
The recommendations listed in this guideline are, whenever possible, evidence based. An extensive evidence review was conducted in April 2014 that included literature published through September 2014. Other selected references published through May 2015 were incorporated by the GWC. Literature included was derived from research involving human subjects, published in English, and indexed in MEDLINE (through PubMed), EMBASE, the Cochrane Library, the Agency for Healthcare Research and Quality, and other selected databases relevant to this guideline. The relevant data are included in evidence tables in the Online Data Supplement. Key search words included but were not limited to the following: ablation therapy (catheter and radiofrequency; fast and slow pathway), accessory pathway (manifest and concealed), antiarrhythmic drugs, atrial fibrillation, atrial tachycardia, atrioventricular nodal reentrant (reentry, reciprocating) tachycardia, atrioventricular reentrant (reentry, reciprocating) tachycardia, beta blockers, calcium channel blockers, cardiac imaging, cardioversion, cost effectiveness, cryotherapy, echocardiography, elderly (aged and older), focal atrial tachycardia, Holter monitor, inappropriate sinus tachycardia, junctional tachycardia, multifocal atrial tachycardia, paroxysmal supraventricular tachycardia, permanent form of junctional reciprocating tachycardia, pre-excitation, pregnancy, quality of life, sinoatrial node, sinus node reentry, sinus tachycardia, supraventricular tachycardia, supraventricular arrhythmia, tachycardia, tachyarrhythmia, vagal maneuvers (Valsalva maneuver), and Wolff-Parkinson-White syndrome. Additionally, the GWC reviewed documents related to supraventricular tachycardia (SVT) previously published by the ACC, AHA, and Heart Rhythm Society (HRS). References selected and published in this document are representative and not all-inclusive.
An independent ERC was commissioned to perform a systematic review of key clinical questions, the results of which were considered by the GWC for incorporation into this guideline. The systematic review report on the management of asymptomatic patients with Wolff-Parkinson-White (WPW) syndrome is published in conjunction with this guideline.9
1.2. Organization of the GWC
The GWC consisted of clinicians, cardiologists, electrophysiologists (including those specialized in pediatrics), and a nurse (in the role of patient representative) and included representatives from the ACC, AHA, and HRS.
1.3. Document Review and Approval
This document was reviewed by 8 official reviewers nominated by the ACC, AHA, and HRS, and 25 individual content reviewers. Reviewers' RWI information was distributed to the GWC and is published in this document (Appendix 2).
This document was approved for publication by the governing bodies of the ACC, the AHA, and the HRS.
1.4. Scope of the Guideline
The purpose of this joint ACC/AHA/HRS document is to provide a contemporary guideline for the management of adults with all types of SVT other than atrial fibrillation (AF). Although AF is, strictly speaking, an SVT, the term SVT generally does not refer to AF. AF is addressed in the 2014 ACC/AHA/HRS Guideline for the Management of Atrial Fibrillation (2014 AF guideline).10 The present guideline addresses other SVTs, including regular narrow–QRS complex tachycardias, as well as other, irregular SVTs (eg, atrial flutter with irregular ventricular response and multifocal atrial tachycardia [MAT]). This guideline supersedes the "2003 ACC/AHA/ESC Guidelines for the Management of Patients With Supraventricular Arrhythmias."11 It incorporates new and existing knowledge derived from published clinical trials, basic science, and comprehensive review articles, along with evolving treatment strategies and new drugs. Some recommendations from the earlier guideline have been updated as warranted by new evidence or a better understanding of existing evidence, whereas other inaccurate, irrelevant, or overlapping recommendations were deleted or modified. Whenever possible, we reference data from the acute clinical care environment; however, in some cases, the reference studies from the invasive electrophysiology laboratory inform our understanding of arrhythmia diagnosis and management. Although this document is aimed at the adult population (≥18 years of age) and offers no specific recommendations for pediatric patients, as per the reference list, we examined literature that included pediatric patients. In some cases, the data from noninfant pediatric patients helped inform this guideline.
In the current healthcare environment, cost consideration cannot be isolated from shared decision making and patient-centered care. The AHA and ACC have acknowledged the importance of value in health care, calling for eventual development of a Level of Value for practice recommendations in the "2014 ACC/AHA Statement on Cost/Value Methodology in Clinical Practice Guidelines and Performance Measures."6 Although quality-of-life and cost-effectiveness data were not sufficient to allow for development of specific recommendations, the GWC agreed the data warranted brief discussion (Sections 10 and 11). Throughout this document, and associated with all recommendations and algorithms, the importance of shared decision making should be acknowledged. Each approach, ranging from observation to drug treatment to ablation, must be considered in the setting of a clear discussion with the patient regarding risk, benefit and personal preference. See Section 12 for additional information.
In developing this guideline, the GWC reviewed prior published guidelines and related statements. Table 2 contains a list of guidelines and statements deemed pertinent to this writing effort and is intended for use as a resource, thus obviating the need to repeat existing guideline recommendations.
| Title | Organization | Publication Year (Reference) |
|---|---|---|
| Guidelines | ||
| Atrial fibrillation | AHA/ACC/HRS | 201410 |
| Stable ischemic heart disease | ACC/AHA/ACP/AATS/ PCNA/SCAI/STS | 201412 201213 |
| Valvular heart disease | AHA/ACC | 201414 |
| Assessment of cardiovascular risk | ACC/AHA | 201315 |
| Heart failure | ACC/AHA | 201316 |
| Antithrombotic therapy for valvular heart disease | ACCP | 201217 |
| Atrial fibrillation | ESC | 201218 201019 |
| Device-based therapy | ACC/AHA/HRS | 201220 |
| Atrial fibrillation | CCS | 201421 201122 |
| Hypertrophic cardiomyopathy | ACC/AHA | 201123 |
| Secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease | AHA/ACC | 201124 |
| Adult congenital heart disease | ACC/AHA | 200825* |
| Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC VII) | NHLBI | 200326 |
| Statements | ||
| Catheter ablation in children and patients with congenital heart disease | PACES/HRS | 2015 (in press)27 |
| Postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope | HRS | 201528 |
| Arrhythmias in adult congenital heart disease | PACES/HRS | 201429 |
| Catheter and surgical ablation of atrial fibrillation | HRS/EHRA/ECAS | 201230 |
| CPR and emergency cardiovascular care | AHA | 201031* |
2. General Principles
2.1. Mechanisms and Definitions
For the purposes of this guideline, SVT is defined as per Table 3, which provides definitions and the mechanism(s) of each type of SVT. The term SVT does not generally include AF, and this document does not discuss the management of AF.
| Arrhythmia/Term | Definition |
|---|---|
| Supraventricular tachycardia (SVT) | An umbrella term used to describe tachycardias (atrial and/or ventricular rates in excess of 100 bpm at rest), the mechanism of which involves tissue from the His bundle or above. These SVTs include inappropriate sinus tachycardia, AT (including focal and multifocal AT), macroreentrant AT (including typical atrial flutter), junctional tachycardia, AVNRT, and various forms of accessory pathway-mediated reentrant tachycardias. In this guideline, the term does not include AF. |
| Paroxysmal supraventricular tachycardia (PSVT) | A clinical syndrome characterized by the presence of a regular and rapid tachycardia of abrupt onset and termination. These features are characteristic of AVNRT or AVRT, and, less frequently, AT. PSVT represents a subset of SVT. |
| Atrial fibrillation (AF) | A supraventricular arrhythmia with uncoordinated atrial activation and, consequently, ineffective atrial contraction. ECG characteristics include: 1) irregular atrial activity, 2) absence of distinct P waves, and 3) irregular R-R intervals (when atrioventricular conduction is present). AF is not addressed in this document. |
| Sinus tachycardia | Rhythm arising from the sinus node in which the rate of impulses exceeds 100 bpm. |
| • Physiologic sinus tachycardia | Appropriate increased sinus rate in response to exercise and other situations that increase sympathetic tone. |
| • Inappropriate sinus tachycardia | Sinus heart rate >100 bpm at rest, with a mean 24-h heart rate >90 bpm not due to appropriate physiological responses or primary causes such as hyperthyroidism or anemia. |
| Atrial tachycardia (AT) | |
| • Focal AT | An SVT arising from a localized atrial site, characterized by regular, organized atrial activity with discrete P waves and typically an isoelectric segment between P waves. At times, irregularity is seen, especially at onset ("warm-up") and termination ("warm-down"). Atrial mapping reveals a focal point of origin. |
| • Sinus node reentry tachycardia | A specific type of focal AT that is due to microreentry arising from the sinus node complex, characterized by abrupt onset and termination, resulting in a P-wave morphology that is indistinguishable from sinus rhythm. |
| • Multifocal atrial tachycardia (MAT) | An irregular SVT characterized by ≥3 distinct P-wave morphologies and/or patterns of atrial activation at different rates. The rhythm is always irregular. |
| Atrial flutter | |
| • Cavotricuspid isthmus–dependent atrial flutter: typical | Macroreentrant AT propagating around the tricuspid annulus, proceeding superiorly along the atrial septum, inferiorly along the right atrial wall, and through the cavotricuspid isthmus between the tricuspid valve annulus and the Eustachian valve and ridge. This activation sequence produces predominantly negative "sawtooth" flutter waves on the ECG in leads 2, 3, and aVF and a late positive deflection in V1. The atrial rate can be slower than the typical 300 bpm (cycle length 200 ms) in the presence of antiarrhythmic drugs or scarring. It is also known as "typical atrial flutter" or "cavotricuspid isthmus–dependent atrial flutter" or "counterclockwise atrial flutter." |
| • Cavotricuspid isthmus–dependent atrial flutter: reverse typical | Macroreentrant AT that propagates around in the direction reverse that of typical atrial flutter. Flutter waves typically appear positive in the inferior leads and negative in V1. This type of atrial flutter is also referred to as "reverse typical" atrial flutter or "clockwise typical atrial flutter." |
| • Atypical or non–cavotricuspid isthmus–dependent atrial flutter | Macroreentrant ATs that do not involve the cavotricuspid isthmus. A variety of reentrant circuits may include reentry around the mitral valve annulus or scar tissue within the left or right atrium. A variety of terms have been applied to these arrhythmias according to the re-entry circuit location, including particular forms, such as "LA flutter" and "LA macroreentrant tachycardia" or incisional atrial re-entrant tachycardia due to re-entry around surgical scars. |
| Junctional tachycardia | A nonreentrant SVT that arises from the AV junction (including the His bundle). |
| Atrioventricular nodal reentrant tachycardia (AVNRT) | A reentrant tachycardia involving 2 functionally distinct pathways, generally referred to as "fast" and "slow" pathways. Most commonly, the fast pathway is located near the apex of Koch's triangle, and the slow pathway inferoposterior to the compact AV node tissue. Variant pathways have been described, allowing for "slow-slow" AVNRT. |
| • Typical AVNRT | AVNRT in which a slow pathway serves as the anterograde limb of the circuit and the fast pathway serves as the retrograde limb (also called "slow-fast AVNRT"). |
| • Atypical AVNRT | AVNRT in which the fast pathway serves as the anterograde limb of the circuit and a slow pathway serves as the retrograde limb (also called "fast-slow AV node reentry") or a slow pathway serves as the anterograde limb and a second slow pathway serves as the retrograde limb (also called "slow-slow AVNRT"). |
| Accessory pathway | For the purpose of this guideline, an accessory pathway is defined as an extranodal AV pathway that connects the myocardium of the atrium to the ventricle across the AV groove. Accessory pathways can be classified by their location, type of conduction (decremental or nondecremental), and whether they are capable of conducting anterogradely, retrogradely, or in both directions. Of note, accessory pathways of other types (such as atriofascicular, nodo-fascicular, nodo-ventricular, and fasciculoventricular pathways) are uncommon and are discussed only briefly in this document (Section 7). |
| • Manifest accessory pathways | A pathway that conducts anterogradely to cause ventricular pre-excitation pattern on the ECG. |
| • Concealed accessory pathway | A pathway that conducts only retrogradely and does not affect the ECG pattern during sinus rhythm. |
| • Pre-excitation pattern | An ECG pattern reflecting the presence of a manifest accessory pathway connecting the atrium to the ventricle. Pre-excited ventricular activation over the accessory pathway competes with the anterograde conduction over the AV node and spreads from the accessory pathway insertion point in the ventricular myocardium. Depending on the relative contribution from ventricular activation by the normal AV nodal/His Purkinje system versus the manifest accessory pathway, a variable degree of pre-excitation, with its characteristic pattern of a short P-R interval with slurring of the initial upstroke of the QRS complex (delta wave), is observed. Pre-excitation can be intermittent or not easily appreciated for some pathways capable of anterograde conduction; this is usually associated with a low-risk pathway, but exceptions occur. |
| • Asymptomatic pre-excitation (isolated pre-excitation) | The abnormal pre-excitation ECG pattern in the absence of documented SVT or symptoms consistent with SVT. |
| • Wolff-Parkinson-White syndrome | Syndrome characterized by documented SVT or symptoms consistent with SVT in a patient with ventricular pre-excitation during sinus rhythm. |
| Atrioventricular reentrant tachycardia (AVRT) | A reentrant tachycardia, the electrical pathway of which requires an accessory pathway, the atrium, atrioventricular node (or second accessory pathway), and ventricle. |
| • Orthodromic AVRT | An AVRT in which the reentrant impulse uses the accessory pathway in the retrograde direction from the ventricle to the atrium, and the AV node in the anterograde direction. The QRS complex is generally narrow or may be wide because of pre-existing bundle-branch block or aberrant conduction. |
| • Antidromic AVRT | An AVRT in which the reentrant impulse uses the accessory pathway in the anterograde direction from the atrium to the ventricle, and the AV node for the retrograde direction. Occasionally, instead of the AV node, another accessory pathway can be used in the retrograde direction, which is referred to as pre-excited AVRT. The QRS complex is wide (maximally pre-excited). |
| Permanent form of junctional reciprocating tachycardia (PJRT) | A rare form of nearly incessant orthodromic AVRT involving a slowly conducting, concealed, usually posteroseptal accessory pathway. |
| Pre-excited AF | AF with ventricular pre-excitation caused by conduction over ≥1 accessory pathway(s). |
2.2. Epidemiology, Demographics, and Public Health Impact
The epidemiology of SVT, including its frequency, patterns, causes, and effects, is imprecisely defined because of incomplete data and failure to discriminate among AF, atrial flutter, and other supraventricular arrhythmias. The best available evidence indicates that the prevalence of SVT in the general population is 2.29 per 1000 persons.32 When adjusted by age and sex in the US population, the incidence of paroxysmal supraventricular tachycardia (PSVT) is estimated to be 36 per 100 000 persons per year.32 There are approximately 89 000 new cases per year and 570 000 persons with PSVT.32 Compared with patients with cardiovascular disease, those with PSVT without any cardiovascular disease are younger (37 versus 69 years; P=0.0002) and have faster PSVT (186 bpm versus 155 bpm; P=0.0006). Women have twice the risk of men of developing PSVT.32 Individuals >65 years of age have >5 times the risk of younger persons of developing PSVT.32
Patients with PSVT who are referred to specialized centers for management with ablation are younger, have an equal sex distribution, and have a low frequency of cardiovascular disease.33–47 The frequency of atrioventricular nodal reentrant tachycardia (AVNRT) is greater in women than in men. This may be due to an actual higher incidence in women, or it may reflect referral bias. In persons who are middle-aged or older, AVNRT is more common, whereas in adolescents, the prevalence may be more balanced between atrioventricular reentrant tachycardia (AVRT) and AVNRT, or AVRT may be more prevalent.32 The relative frequency of tachycardia mediated by an accessory pathway decreases with age. The incidence of manifest pre-excitation or WPW pattern on ECG tracings in the general population is 0.1% to 0.3%. However, not all patients with manifest ventricular pre-excitation develop PSVT.47–49 The limited data on the public health impact of SVT indicate that the arrhythmia is commonly a reason for emergency department and primary care physician visits but is infrequently the primary reason for hospital admission.11,50,51
2.3. Evaluation of the Patient With Suspected or Documented SVT
2.3.1. Clinical Presentation and Differential Diagnosis on the Basis of Symptoms
Patients seen in consultation for palpitations often describe symptoms with characteristic features suggestive of SVT that may guide physicians to appropriate testing and a definitive diagnosis. The diagnosis of SVT is often made in the emergency department, but it is common to elicit symptoms suggestive of SVT before initial electrocardiogram/electrocardiographic (ECG) documentation. SVT symptom onset often begins in adulthood; in 1 study in adults, the mean age of symptom onset was 32±18 years of age for AVNRT, versus 23±14 years of age for AVRT.52 In contrast, in a study conducted in pediatric populations, the mean ages of symptom onset of AVRT and AVNRT were 8 and 11 years, respectively.53 In comparison with AVRT, patients with AVNRT are more likely to be female, with an age of onset >30 years.49,54–56 AVNRT onset has been reported after the age of 50 years in 16% and before the age of 20 years in 18%.57 Among women with SVT and no other cardiovascular disease, the onset of symptoms occurred during childbearing years (eg, 15 to 50 years) in 58%.32 The first onset of SVT occurred in only 3.9% of women during pregnancy, but among women with an established history of SVT, 22% reported that pregnancy exacerbated their symptoms.58
SVT has an impact on quality of life, which varies according to the frequency of episodes, the duration of SVT, and whether symptoms occur not only with exercise but also at rest.53,59 In 1 retrospective study in which the records of patients <21 years of age with WPW pattern on the ECG were reviewed, 64% of patients had symptoms at presentation, and an additional 20% developed symptoms during follow-up.60 Modes of presentation included documented SVT in 38%, palpitations in 22%, chest pain in 5%, syncope in 4%, AF in 0.4%, and sudden cardiac death (SCD) in 0.2%.60 Although this was a pediatric population, it provided symptom data that are likely applicable to adults. A confounding factor in diagnosing SVT is the need to differentiate symptoms of SVT from symptoms of panic and anxiety disorders or any condition of heightened awareness of sinus tachycardia (such as postural orthostatic tachycardia syndrome). In 1 study, the criteria for panic disorder were fulfilled in 67% of patients with SVT that remained unrecognized after their initial evaluation. Physicians attributed symptoms of SVT to panic, anxiety, or stress in 54% of patients, with women more likely to be mislabeled with panic disorder than men.61
When AVNRT and AVRT are compared, symptoms appear to differ substantially. Patients with AVNRT more frequently describe symptoms of "shirt flapping" or "neck pounding"54,62 that may be related to pulsatile reversed flow when the right atrium contracts against a closed tricuspid valve (cannon a-waves). During 1 invasive study of patients with AVNRT and AVRT, both arrhythmias decreased arterial pressure and increased left atrial pressure, but simulation of SVT mechanism by timing the pacing of the atria and ventricles showed significantly higher left atrial pressure in simulated AVNRT than in simulated AVRT.62 Polyuria is particularly common with AVNRT and is related to higher right atrial pressures and elevated levels of atrial natriuretic protein in patients with AVNRT compared with patients who have AVRT or atrial flutter.63
True syncope is infrequent with SVT, but complaints of light-headedness are common. In patients with WPW syndrome, syncope should be taken seriously but is not necessarily associated with increased risk of SCD.64 The rate of AVRT is faster when AVRT is induced during exercise,65 yet the rate alone does not explain symptoms of near-syncope. Elderly patients with AVNRT are more prone to syncope or near-syncope than are younger patients, but the tachycardia rate is generally slower in the elderly.66,67 The drop in blood pressure (BP) during SVT is greatest in the first 10 to 30 seconds and somewhat normalizes within 30 to 60 seconds, despite minimal changes in rate.68,69 Shorter ventriculoatrial intervals are associated with a greater mean decrease in BP.69 Studies have demonstrated a relationship between hemodynamic changes and the relative timing of atrial and ventricular activation. In a study of patients with AVNRT with short versus long ventriculoatrial intervals, there was no significant difference in tachycardia cycle length70; however, the induction of typical AVNRT caused a marked initial fall in systemic BP, followed by only partial recovery that resulted in stable hypotension and a reduction in cardiac output due to a decrease in stroke volume. In comparison, atypical AVNRT, having a longer ventriculoatrial interval, exhibited a lesser degree of initial hypotension, a complete recovery of BP, and no significant change in cardiac output.70
The contrasting hemodynamic responses without significant differences in heart rate during SVT confirm that rate alone does not account for these hemodynamic changes. Atrial contraction on a closed valve might impair pulmonary drainage and lead to neural factors that account for these observations. These findings were confirmed in a study performed in the electrophysiological (EP) laboratory: When pacing was used to replicate the timing of ventricular and atrial activation during SVT, the decrease in BP was greatest with simultaneous ventriculoatrial timing, smaller with a short vertriculoatrial interval, and smallest with a long ventriculoatrial interval.71 An increase in central venous pressure followed the same trend. Sympathetic nerve activity increased with all 3 pacing modalities but was most pronounced with simultaneous atrial and ventricular pacing or a short ventriculoatrial interval.
In a study of the relationship of SVT with driving, 57% of patients with SVT experienced an episode while driving, and 24% of these considered it to be an obstacle to driving.72 This sentiment was most common in patients who had experienced syncope or near-syncope. Among patients who experienced SVT while driving, 77% felt fatigue, 50% had symptoms of near-syncope, and 14% experienced syncope. Women had more symptoms in each category.
See Online Data Supplement 1 for additional data on clinical presentation and differential diagnosis on the basis of symptoms.
2.3.2. Evaluation of the ECG
Figures 1 to 6 provide representative ECGs, with Figure 1 showing ventricular tachycardia (VT) and Figures 2 to 5 showing some of the most common types of these SVTs.

- Download figure
- Download PowerPoint
Figure 1. ECG showing AV dissociation during VT in a patient with a wide–QRS complex tachycardia. *P waves are marked with arrows. AV indicates atrioventricular; ECG, electrocardiogram; and VT, ventricular tachycardia. Reproduced with permission from Blomström-Lundqvist et al.11

- Download figure
- Download PowerPoint
Figure 2. Typical AVNRT and normal sinus rhythm after conversion. Upper panel: The arrow points to the P waves, which are inscribed at the end of the QRS complex, seen best in the inferior leads and as a slightly positive R′ (pseudo r prime) in lead V1. The reentrant circuit involves anterograde conduction over a slow atrioventricular node pathway, followed by retrograde conduction in a fast atrioventricular node pathway. Typical AVNRT is a type of short RP tachycardia. Middle panel: When the patient is in sinus rhythm, the arrow indicates where the R′ is absent in V1. Bottom panels: Magnified portions of lead V1 in AVNRT (left) and sinus rhythm (right) are shown. AVNRT indicates atrioventricular nodal reentrant tachycardia.

- Download figure
- Download PowerPoint
Figure 3. Atypical AVNRT. Arrows point to the P wave. The reentrant circuit involves anterograde conduction over a fast atrioventricular node pathway, followed by retrograde conduction in a slow atrioventricular node pathway, resulting in a retrograde P wave (negative polarity in inferior leads) with long RP interval. This ECG does not exclude PJRT or a low septal atrial tachycardia, which can appear very similar to this ECG. AVNRT indicates atrioventricular nodal reentrant tachycardia; ECG, electrocardiogram; and PJRT, permanent form of junctional reciprocating tachycardia.

- Download figure
- Download PowerPoint
Figure 4. Orthodromic atrioventricular reentrant tachycardia. Arrows point to the P waves, which are inscribed in the ST-segment after the QRS complex. The reentrant circuit involves anterograde conduction over the atrioventricular node, followed by retrograde conduction over an accessory pathway, which results in a retrograde P wave with short RP interval.

- Download figure
- Download PowerPoint
Figure 5. Atrial tachycardia. Arrows point to the P wave, which is inscribed before the QRS complex. The focus of this atrial tachycardia was mapped during electrophysiological study to an area near the left inferior pulmonary vein.

- Download figure
- Download PowerPoint
Figure 6. Permanent form of junctional reciprocating tachycardia (PJRT). Tachycardia starts after 2 beats of sinus rhythm. Arrows point to the P wave, which is inscribed before the QRS complex. The reentrant circuit involves anterograde conduction over the atrioventricular node, followed by retrograde conduction over a slowly conducting (or decremental) accessory pathway, usually located in the posteroseptal region, to provide a retrograde P wave with long RP interval. This ECG does not exclude atypical AVNRT or a low septal atrial tachycardia, which can appear very similar to this ECG. AVNRT indicates atrioventricular nodal reentrant tachycardia; ECG, electrocardiogram; and PJRT, permanent form of junctional reciprocating tachycardia.
A 12-lead ECG obtained during tachycardia and during sinus rhythm may reveal the etiology of tachycardia. For the patient who describes prior, but not current, symptoms of palpitations, the resting ECG can identify pre-excitation that should prompt a referral to a cardiac electrophysiologist.
A wide-complex tachycardia (QRS duration >120 ms) may represent either VT or a supraventricular rhythm with abnormal conduction. Conduction abnormalities may be due to rate-related aberrant conduction, pre-existing bundle-branch block seen in sinus rhythm, or an accessory pathway that results in pre-excitation (Table 4). The presence of atrioventricular (AV) dissociation (with ventricular rate faster than atrial rate) or fusion complexes–representing dissociation of supraventricular impulses from a ventricular rhythm– provides the diagnosis of VT (Figure 1). Other criteria are useful but not diagnostic. Concordance of the precordial QRS complexes such that all are positive or negative suggests VT or pre-excitation, whereas QRS complexes in tachycardia that are identical to those seen in sinus rhythm are consistent with SVT. Other, more complicated ECG algorithms have been developed to distinguish VT from SVT, such as the Brugada criteria, which rely on an examination of the QRS morphology in the precordial leads,73 and the Vereckei algorithm, which is based on an examination of the QRS complex in lead aVR74 (Table 5). The failure to correctly identify VT can be potentially life threatening, particularly if misdiagnosis results in VT being treated with verapamil or diltiazem. Adenosine is suggested in the "2010 AHA Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care–Part 8: Adult Advanced Cardiovascular Life Support" (2010 Adult ACLS guideline)75 if a wide-complex tachycardia is monomorphic, regular, and hemodynamically tolerated, because adenosine may help convert the rhythm to sinus and may help in the diagnosis. When doubt exists, it is safest to assume any wide-complex tachycardia is VT, particularly in patients with known cardiovascular disease, such as prior myocardial infarction.
| Mechanism |
|---|
| Ventricular tachycardia |
| SVT with pre-existing bundle-branch block or intraventricular conduction defect |
| SVT with aberrant conduction due to tachycardia (normal QRS when in sinus rhythm) |
| SVT with wide QRS related to electrolyte or metabolic disorder |
| SVT with conduction over an accessory pathway (pre-excitation) |
| Paced rhythm |
| Artifact |
| Findings or Leads on ECG Assessed | Interpretation |
|---|---|
| QRS complex in leads V1-V6 (Brugada criteria)73 | • Lack of any R-S complexes implies VT |
| • R-S interval (onset of R wave to nadir of S wave) >100 ms in any precordial lead implies VT | |
| QRS complex in aVR (Vereckei algorithm)74 | • Presence of initial R wave implies VT |
| • Initial R or Q wave >40 ms implies VT | |
| • Presence of a notch on the descending limb at the onset of a predominantly negative QRS implies VT | |
| AV dissociation* | • Presence of AV dissociation (with ventricular rate faster than atrial rate) or fusion complexes implies VT |
| QRS complexes in precordial leads all positive or all negative (concordant) | • Implies VT |
| QRS in tachycardia that is identical to sinus rhythm78 | • Suggests SVT |
| R-wave peak time in lead II78 | • R-wave peak time ≥50 ms suggests VT |
For a patient presenting in SVT, the 12-lead ECG can potentially identify the arrhythmia mechanism (Figure 7). The tachycardia should first be classified according to whether there is a regular or irregular ventricular rate. An irregular ventricular rate suggests AF, MAT, or atrial flutter with variable AV conduction. When AF is associated with a rapid ventricular response, the irregularity of the ventricular response is less easily detected and can be misdiagnosed as a regular SVT.76 If the atrial rate exceeds the ventricular rate, then atrial flutter or AT (focal or multifocal) is usually present (rare cases of AVNRT with 2:1 conduction have been described77).

- Download figure
- Download PowerPoint
Figure 7. Differential diagnosis for adult narrow QRS tachycardia. Patients with junctional tachycardia may mimic the pattern of slow-fast AVNRT and may show AV dissociation and/or marked irregularity in the junctional rate. *RP refers to the interval from the onset of surface QRS to the onset of visible P wave (note that the 90-ms interval is defined from the surface ECG,79 as opposed to the 70-ms ventriculoatrial interval that is used for intracardiac diagnosis 80). AV indicates atrioventricular; AVNRT, atrioventricular nodal reentrant tachycardia; AVRT, atrioventricular reentrant tachycardia; ECG, electrocardiogram; MAT, multifocal atrial tachycardia; and PJRT, permanent form of junctional reentrant tachycardia. Modified with permission from Blomström-Lundqvist et al.11
If the SVT is regular, this may represent AT with 1:1 conduction or an SVT that involves the AV node. Junctional tachycardias, which originate in the AV junction (including the His bundle), can be regular or irregular, with variable conduction to the atria. SVTs that involve the AV node as a required component of the tachycardia reentrant circuit include AVNRT (Section 6: Figures 2 and 3) and AVRT (Section 7: Figures 4 and 6). In these reentrant tachycardias, the retrogradely conducted P wave may be difficult to discern, especially if bundle-branch block is present. In typical AVNRT, atrial activation is nearly simultaneous with the QRS, so the terminal portion of the P wave is usually located at the end of the QRS complex, appearing as a narrow and negative deflection in the inferior leads (a pseudo S wave) and a slightly positive deflection at the end of the QRS complex in lead V1 (pseudo R′). In orthodromic AVRT (with anterograde conduction down the AV node), the P wave can usually be seen in the early part of the ST-T segment. In typical forms of AVNRT and AVRT, because the P wave is located closer to the prior QRS complex than the subsequent QRS complex, the tachycardias are referred to as having a "short RP." They also have a 1:1 relationship between the P wave and QRS complex, except in rare cases of AVNRT in which 2:1 AV block or various degrees of AV block can occur. In unusual cases of AVNRT (such as "fast-slow"), the P wave is closer to the subsequent QRS complex, providing a long RP. The RP is also long during an uncommon form of AVRT, referred to as the permanent form of junctional reciprocating tachycardia (PJRT), in which an unusual accessory bypass tract with "decremental" (slowly conducting) retrograde conduction during orthodromic AVRT produces delayed atrial activation and a long RP interval.
A long RP interval is typical of AT because the rhythm is driven by the atrium and conducts normally to the ventricles. In AT, the ECG will typically show a P wave with a morphology that differs from sinus that is usually seen near the end of or shortly after the T wave (Figure 5). In sinus node reentry tachycardia, a form of focal AT, the P-wave morphology is identical to the P wave in sinus rhythm.
2.4. Principles of Medical Therapy
See Figure 8 for the algorithm for acute treatment of tachycardia of unknown mechanism; Figure 9 for the algorithm for ongoing management of tachycardia of unknown mechanism; Table 6 for acute drug therapy for SVT (intravenous administration); and Table 7 for ongoing drug therapy for SVT (oral administration).
| Drug | Initial Dose | Subsequent or Maintenance Dose | Potential Adverse Effects | Precautions (Exclude or Use With Caution) and Interactions |
|---|---|---|---|---|
| Nucleoside | ||||
| Adenosine | 6-mg rapid IV bolus (injected into IV as proximal or as close to the heart as possible), administered over 1–2 s, followed by rapid saline flush | If no result within 1–2 min, 12-mg rapid IV bolus; can repeat 12-mg dose 1 time. The safe use of 18-mg bolus doses has been reported.117 | Transient AV block, flushing, chest pain, hypotension, or dyspnea, AF can be initiated or cause decompensation in the presence of pre-excitation, PVCs/ventricular tachycardia, bronchospasm (rare), or coronary steal. Minor side effects are usually transient because of adenosine's very short half-life. | • AV block greater than first degree or SA node dysfunction (in absence of pacemaker) |
| • Reactive airway disease | ||||
| • Concomitant use of verapamil or digoxin | ||||
| • WPW | ||||
| Beta blockers | ||||
| Esmolol | 500-mcg/kg IV bolus over 1 min | Infusion at 50–300 mcg/kg/min, with repeat boluses between each dosing increase | Hypotension, worsening HF, bronchospasm, bradycardia | • AV block greater than first degree or SA node dysfunction (in absence of pacemaker) |
| • Decompensated systolic HF | ||||
| • Hypotension | ||||
| • Cardiogenic shock | ||||
| • Reactive airway disease | ||||
| • Renal dysfunction | ||||
| • Drugs with SA and/or AV nodal–blocking properties | ||||
| Metoprolol tartrate | 2.5–5.0-mg IV bolus over 2 min | Can repeat 2.5- to 5.0-mg IV bolus in 10 min, up to 3 doses | Hypotension, worsening HF, bronchospasm, bradycardia | • AV block greater than first degree or SA node dysfunction (in absence of pacemaker) |
| • Decompensated systolic HF | ||||
| • Hypotension | ||||
| • Reactive airway disease | ||||
| • Drugs with SA and/or AV nodal–blocking properties | ||||
| Propranolol | 1 mg IV over 1 min | Can repeat 1 mg IV at 2-min intervals, up to 3 doses | Hypotension, worsening HF, bronchospasm, bradycardia | • AV block greater than first degree or SA node dysfunction (in absence of pacemaker) |
| • Cardiogenic shock | ||||
| • Reactive airway disease | ||||
| • Decompensated HF | ||||
| • Hypotension | ||||
| • Hepatic or renal dysfunction | ||||
| • Drugs with SA and/or AV nodal–blocking properties | ||||
| Nondihydropyridine calcium channel antagonists | ||||
| Diltiazem | 0.25-mg/kg IV bolus over 2 min | Infusion at 5–10 mg/h, up to 15 mg/h | Hypotension, worsening HF in patients with pre-existing ventricular dysfunction, bradycardia, abnormal liver function studies, acute hepatic injury (rare) | • AV block greater than first degree or SA node dysfunction (in absence of pacemaker) |
| • WPW with AF/atrial flutter | ||||
| • Hypotension† | ||||
| • Decompensated systolic HF/LV dysfunction | ||||
| • Drugs with SA and/or AV nodal–blocking properties | ||||
| • Hepatic or renal dysfunction | ||||
| • Diltiazem is a substrate of CYP3A4 (major) and a moderate CYP3A4 inhibitor | ||||
| • Apixaban, itraconazole, bosutinib, ceritinib, cilostazol, cyclosporine, everolimus, ibrutinib, idelalisib, ivabradine, lomitapide, olaparib, posaconazole, ranolazine, rifampin, simeprevir, voriconazole | ||||
| Verapamil | 5–10-mg (0.075–0. 15-mg/kg) IV bolus over 2 min | If no response, can give an additional 10 mg (0.15 mg/kg) 30 min after first dose; then infusion at 0.005 mg/kg/min | Hypotension, worsening HF in patients with pre-existing ventricular dysfunction, pulmonary edema in patients with hypertrophic cardiomyopathy, bradycardia | • AV block greater than first degree or SA node dysfunction (in absence of pacemaker) |
| • Decompensated systolic HF/LV dysfunction | ||||
| • Drugs with SA and/or AV nodal–blocking properties | ||||
| • Hypotension† | ||||
| • Cardiogenic shock | ||||
| • WPW with AF/atrial flutter | ||||
| • Hepatic or renal dysfunction | ||||
| • Verapamil is a moderate CYP3A4 inhibitor and also inhibits P-glycoprotein | ||||
| • Contraindicated with dofetilide | ||||
| • Itraconazole, bosutinib, ceritinib, cilostazol, colchicine, cyclosporine, everolimus, dabigatran, edoxaban, flecainide, ibrutinib, ivabradine, olaparib, posaconazole, ranolazine, rivaroxaban, rifampin, silodosin, simeprevir, rivaroxaban, rifampin, simvastatin, topotecan, trabectedin, vincristine, voriconazole, grapefruit juice | ||||
| Cardiac glycosides | ||||
| Digoxin | 0.25–0.5-mg IV bolus | Can repeat 0.25-mg IV bolus, up to maximum dose of 1.0 mg over 24 h (ie, maximum loading dose 8–12 mcg/kg), given at 6–8-h intervals; maintenance dose based on patient's age, lean body weight, renal function, and concomitant drugs (IV 2.4–3.6 mcg/kg/d) | Anorexia, nausea, vomiting, visual changes and cardiac arrhythmias if digoxin toxicity (associated with levels >2 ng/mL, although symptoms may also occur at lower levels) | • Renal dysfunction |
| • WPW with AF/atrial flutter | ||||
| • AV block greater than first degree or SA node dysfunction (in absence of pacemaker) | ||||
| • Drugs with AV nodal-blocking properties | ||||
| • Digoxin is a P-glycoprotein substrate | ||||
| • Dronedarone (reduce dose by at least 50%), amiodarone (reduce dose by 30%–50%) | ||||
| • Verapamil, clarithromycin, cyclosporine, erythromycin, flecainide, itraconazole, posaconazole, propafenone, voriconazole: Monitor digoxin levels | ||||
| • A large retrospective study suggested an increased risk in mortality in patients who were treated with digoxin for newly diagnosed AF or atrial flutter; although the data were collected from a population that was different from SVT patients, digoxin should be used with caution.118 | ||||
| Class III antiarrhythmic agents | ||||
| Amiodarone | 150 mg IV over 10 min | Infusion at 1 mg/min (360 mg) over next 6 h; then 0.5 mg/min (540 mg) over remaining 18 h | Hypotension, bradycardia, phlebitis, QT prolongation, torsades de pointes (rare), increased INR | • Sinus or AV conduction disease (in absence of pacemaker) |
| • Inflammatory lung disease (acute) | ||||
| • Hepatic dysfunction | ||||
| • Drugs with SA and/or AV nodal–blocking properties | ||||
| • Amiodarone is a substrate of and inhibits p-glycoprotein and CYP2C9 (moderate), CYP2D6 (moderate), and CYP3A4 (weak); amiodarone is a substrate for CYP3A4 (major) and CYP2C8 (major);amiodarone is an inhibitor of OCT2 | ||||
| • Reduce warfarin dose by 50% and reduce digoxin dose by 30%–50% | ||||
| • Agalsidase alfa, agalsidase beta, azithromycin, bosutinib, ceritinib, colchicine, dabigatran, edoxaban, flecainide, ivabradine, ledipasvir/sofosbuvir, lopinavir, lopinavir/ritonavir, lovastatin, nelfinavir, pazopanib, propafenone, simvastatin, ritonavir, rivaroxaban, saquinavir, sofosbuvir, topotecan, vincristine, grapefruit juice | ||||
| Ibutilide | Contraindicated when QTc >440 ms‡; 1 mg over 10 min (if ≥60 kg); if <60 kg, then 0.01 mg/kg | Can repeat 1 mg once, if the arrhythmia does not terminate within 10 min§ | QT prolongation, torsades de pointes, AV block | • Prolonged QT interval |
| • History of torsades de pointes | ||||
| • Avoid other QT interval–prolonging drugs | ||||
| • Concurrent administration of high-dose magnesium has been associated with enhanced efficacy and safety119,120 | ||||
| Drug | Initial Daily Dose(s) | Maximum Total Daily Maintenance Dose | Potential Adverse Effects | Precautions (Exclude or Use With Caution) and Interactions |
|---|---|---|---|---|
| Beta blockers | ||||
| Atenolol | 25–50 mg QD | 100 mg QD (reduced dosing in patients with severe renal dysfunction) | Hypotension, bronchospasm, bradycardia | • AV block greater than first degree or SA node dysfunction (in absence of pacemaker) |
| • Decompensated systolic HF | ||||
| • Hypotension | ||||
| • Reactive airway disease | ||||
| • Severe renal dysfunction | ||||
| • Drugs with SA and/or AV nodal–blocking properties | ||||
| Metoprolol tartrate | 25 mg BID | 200 mg BID | Hypotension, bronchospasm, bradycardia | • AV block greater than first degree or SA node dysfunction (in absence of pacemaker) |
| • Decompensated systolic HF | ||||
| • Hypotension | ||||
| • Reactive airway disease | ||||
| • Drugs with SA and/or AV nodal–blocking properties | ||||
| Metoprolol succinate (long-acting) | 50 mg QD | 400 mg QD | Hypotension, bronchospasm, bradycardia | • AV block greater than first degree or SA node dysfunction (in absence of pacemaker) |
| • Decompensated systolic HF | ||||
| • Hypotension | ||||
| • Reactive airway disease | ||||
| • Drugs with SA and/or AV nodal–blocking properties | ||||
| Nadolol | 40 mg QD | 320 mg QD (reduced dosage with renal impairment) | Hypotension, bronchospasm, bradycardia | • AV block greater than first degree or SA node dysfunction (in absence of pacemaker) |
| • Reactive airway disease | ||||
| • Cardiogenic shock | ||||
| • Decompensated HF | ||||
| • Hypotension | ||||
| • Renal dysfunction | ||||
| • Drugs with SA and/or AV nodal–blocking properties | ||||
| Propranolol | 30–60 mg in divided or single dose with long-acting formulations | 40–160 mg in divided or single dose with long-acting formulations | Hypotension, worsening HF, bronchospasm, bradycardia | • AV block greater than first degree or SA node dysfunction (in absence of pacemaker) |
| • Reactive airway disease | ||||
| • Decompensated systolic HF | ||||
| • Hypotension | ||||
| • Drugs with SA and/or AV nodal–blocking properties | ||||
| Nondihydropyridine calcium channel antagonists | ||||
| Diltiazem | 120 mg daily in divided or single dose with long-acting formulations | 360 mg daily in divided or single dose with long-acting formulations | Hypotension, worsening HF in patients with pre-existing ventricular dysfunction, bradycardia, abnormal liver function studies, acute hepatic injury (rare) | • AV block greater than first degree or SA node dysfunction (in absence of pacemaker) |
| • Hypotension† | ||||
| • Decompensated systolic HF/severe LV dysfunction | ||||
| • WPW with AF/atrial flutter | ||||
| • Drugs with SA and/or AV nodal–blocking properties | ||||
| • Diltiazem is a substrate of CYP3A4 (major) and a moderate CYP3A4 inhibitor | ||||
| • Apixaban, itraconazole, bosutinib, ceritinib, cilostazol, cyclosporine, everolimus, ibrutinib, idelalisib, ivabradine, lomitapide, olaparib, ranolazine, rifampin, simeprevir | ||||
| Verapamil | 120 mg daily in divided or single dose with long-acting formulations | 480 mg daily in divided or single dose with long-acting formulations | Hypotension, worsening HF in patients with pre-existing ventricular dysfunction, pulmonary edema in patients with hypertrophic cardiomyopathy, bradycardia, abnormal liver function studies | • AV block greater than first degree or SA node dysfunction (in absence of pacemaker) |
| • Decompensated systolic HF/severe LV dysfunction | ||||
| • Hypotension† | ||||
| • WPW with AF/atrial flutter | ||||
| • Verapamil is a moderate CYP3A4 inhibitor and also inhibits P-glycoprotein | ||||
| • Contraindicated with dofetilide | ||||
| • Itraconazole, bosutinib, ceritinib, cilostazol, colchicine, cyclosporine, everolimus, dabigatran, edoxaban, flecainide, ibrutinib, ivabradine, olaparib, ranolazine, rivaroxaban, rifampin, silodosin, simeprevir, rivaroxaban, rifampin, simvastatin, topotecan, trabectedin, vincristine, grapefruit juice | ||||
| Cardiac glycosides | ||||
| Digoxin | Loading: 0.5 mg, with additional 0.125–0.25-mg doses administered at 6–8-h intervals until evidence of adequate effect (maximum dose 8–12 mcg/kg over 24 h) | 0.25 mg QD Maintenance: 0.125–0.25 mg QD, with dosing based on patient's age, lean body weight, and renal function and drug interactions; occasionally down to 0.0625 mg in cases of renal impairment (trough serum digoxin level 0.5 to 1 ng/mL) | Bradycardia, heart block, anorexia, nausea, vomiting, visual changes and cardiac arrhythmias in cases of digoxin toxicity (associated with levels >2 ng/mL, although symptoms may also occur at lower levels) | • Renal dysfunction |
| • WPW with AF/atrial flutter | ||||
| • AV block greater than first degree or SA node dysfunction (in absence of pacemaker) | ||||
| • Drugs with SA and/or AV nodal–blocking properties | ||||
| • Reduce dose by 30%–50% when administering with amiodarone and by 50% when administering with dronedarone | ||||
| • Monitor digoxin concentrations with verapamil, clarithromycin, erythromycin, itraconazole, cyclosporine, propafenone, flecainide | ||||
| Class Ic antiarrhythmic agents | ||||
| Flecainide | 50 mg every 12 h | 150 mg every 12 h (PR and QRS intervals should be monitored. May consider monitoring flecainide plasma levels, keeping trough plasma levels below 0.7–1.0 mcg/mL) | Atrial flutter with 1:1 AV conduction‡, QT prolongation, torsades de pointes, worsening HF, bradycardia | • Sinus or AV conduction disease (in absence of pacemaker) |
| • Cardiogenic shock | ||||
| • Avoid in structural heart disease (including ischemic heart disease) | ||||
| • Atrial flutter (unless concomitant AV nodal therapy to avoid 1:1 conduction) | ||||
| • Brugada syndrome | ||||
| • Renal dysfunction | ||||
| • Hepatic dysfunction | ||||
| • QT-prolonging drugs | ||||
| • Amiodarone, digoxin, ritonavir, saquinavir, tipranavir | ||||
| Propafenone | 150 mg every 8 h (immediate release); 225 mg every 12 h (extended release) | 300 mg every 8 h (immediate release); 425 mg every 12 h (extended release) (PR and QRS interval should be monitored. Consider dosage reduction with hepatic impairment) | Atrial flutter with 1:1 AV conduction‡, QT prolongation, torsades de pointes, bradycardia, bronchospasm | • Sinus or AV conduction disease (in absence of pacemaker) |
| • Cardiogenic shock | ||||
| • Hypotension | ||||
| • Reactive airway disease Avoid in structural heart disease (including ischemic heart disease) | ||||
| • Atrial flutter (unless concomitant AV nodal therapy to avoid 1:1 conduction) | ||||
| • Brugada syndrome | ||||
| • Hepatic dysfunction | ||||
| • QT-prolonging drugs | ||||
| • Drugs with SA and/or AV nodal–blocking properties | ||||
| • Amiodarone, ritonavir, saquinavir, tipranavir | ||||
| Class III antiarrhythmic agents | ||||
| Amiodarone | 400–600 mg QD in divided doses for 2–4 wk (loading dose); followed by 100–200 mg QD (maintenance dose) | Up to 1200 mg QD may be considered in an inpatient monitoring setting (loading dose); up to 200 mg QD maintenance (to minimize long-term adverse effects) | Bradycardia, QT prolongation, torsades de pointes (rare), gastrointestinal upset, constipation, hypothyroidism, hyperthyroidism, pulmonary fibrosis, hepatic toxicity, corneal deposits, optic neuritis, peripheral neuropathy, photosensitivity, adult respiratory distress syndrome after cardiac or noncardiac surgery (rare) | • Sinus or AV conduction disease (in absence of pacemaker) |
| • Inflammatory lung disease | ||||
| • Hepatic dysfunction | ||||
| • Hypothyroidism, hyperthyroidism | ||||
| • Peripheral neuropathy | ||||
| • Abnormal gait/ataxia | ||||
| • Optic neuritis | ||||
| • Drugs with SA and/or AV nodal–blocking properties | ||||
| • Amiodarone is a substrate of and inhibits P-glycoprotein and CYP2C9 (moderate), CYP2D6 (moderate), and CYP3A4 (weak); amiodarone is a substrate for CYP3A4 (major) and CYP2C8 (major); amiodarone is an inhibitor of OCT2 | ||||
| • Reduce warfarin dose by 50%, and reduce digoxin dose by 30%–50% | ||||
| • Agalsidase alfa, agalsidase beta, azithromycin, bosutinib, ceritinib, colchicine, dabigatran, edoxaban, flecainide, ivabradine, ledipasvir/sofosbuvir, lopinavir, lopinavir/ritonavir, lovastatin, nelfinavir, pazopanib, propafenone, simvastatin, ritonavir, rivaroxaban, saquinavir, sofosbuvir, topotecan, vincristine, grapefruit juice | ||||
| Dofetilide | 500 mcg every 12 h (if CrCl >60 mL/min)250 mcg every 12 h (if CrCl 40–60 mL/min)125 mcg every 12 h (if CrCl 20 to <40 mL/min)Not recommended if CrCl <20 mL/minAdjust dose for renal function, body size, and ageInitiate for minimum of 3 d in a facility that can provide continuous ECG monitoring and cardiac resuscitationContraindicated if the baseline QTc interval or QTc >440 ms† or 500 ms in patients with ventricular conduction abnormalities | Repeat ECG 2–3 h after administering the first dose to determine QTc; if the QTc increased by >15% compared with baseline or if QTc is >500 ms† (550 ms in patients with ventricular conduction abnormalities), subsequent dosing should be down titrated by 50%; at 2–3 h after each subsequent dose, determine QTc (for in-hospital doses 2–5); if at any time after the second dose the QTc is >500 ms‖ (550 ms in patients with ventricular conduction abnormalities), dofetilide should be discontinued | QT prolongation, torsades de pointes | • Severe renal dysfunction (contraindicated if CrCl <20 mL/min) |
| • Prolonged QT | ||||
| • History of torsades de pointes | ||||
| • Concomitant use of hydrochlorothiazide, cimetidine, dolutegravir, itraconazole, ketoconazole, megestrol, trimethoprim, prochlorperazine trimethoprim/sulfamethoxazole or verapamil, contraindicated | ||||
| • Avoid other QT-prolonging drugs | ||||
| Sotalol | 40–80 mg every 12 h (Patients initiated or reinitiated on sotalol should be placed in a facility that can provide cardiac resuscitation and continuous electrocardiographic monitoring for a minimum of 3 d). Contraindicated if the QTc† interval is >450 ms.CrCl should be calculated before dosing. If CrCl >60 mL/min, then dosing frequency is twice daily. If CrCl 40–60 mL/min, dosing interval is every 24 h. If CrCl <40 mL/min, should not be used.) | 160 mg every 12 h (During initiation and titration, the QT interval should be monitored 2–4 h after each dose. If the QT interval prolongs to ≥500 ms, the dose must be reduced or the drug discontinued.) | QT prolongation, torsades de pointes, bradycardia, bronchospasm | • Prolonged QT |
| • Renal dysfunction | ||||
| • Hypokalemia | ||||
| • Diuretic therapy | ||||
| • Avoid other QT-prolonging drugs | ||||
| • Sinus or AV nodal dysfunction (in absence of pacemaker) | ||||
| • Decompensated systolic HF | ||||
| • Cardiogenic shock | ||||
| • Reactive airway disease | ||||
| • Drugs with SA and/or AV–nodal blocking properties | ||||
| Miscellaneous | ||||
| Ivabradine | 5 mg BID | 7.5 mg BID | Phosphenes, AF | • Concomitant drugs that can exacerbate bradycardia |
| • Contraindicated in decompensated HF | ||||
| • Contraindicated if BP <90/50 mm Hg | ||||
| • Contraindicated in severe hepatic impairment | ||||
| • Hypertension | ||||
| • Ivabradine is a substrate of CYP3A4 (major) | ||||
| • Avoid use with concomitant strong CYP3A4 inhibitors (boceprevir, clarithromycin, indinavir, itraconazole, lopinavir/ritonavir, nelfinavir, ritonavir, saquinavir, telaprevir, posaconazole, voriconazole) | ||||
| • Avoid use with strong CYP3A4 inducers (carbamazepine, phenytoin, rifampin, St. John's wort) | ||||
| • Avoid use with diltiazem, verapamil, grapefruit juice | ||||

- Download figure
- Download PowerPoint
Figure 8. Acute treatment of regular SVT of unknown mechanism. Colors correspond to Class of Recommendation in Table 1; drugs listed alphabetically. *For rhythms that break or recur spontaneously, synchronized cardioversion is not appropriate. IV indicates intravenous; and SVT, supraventricular tachycardia.

- Download figure
- Download PowerPoint
Figure 9. Ongoing management of SVT of unknown mechanism. Colors correspond to Class of Recommendation in Table 1; drugs listed alphabetically. *Clinical follow-up without treatment is also an option. EP indicates electrophysiological; pt, patient; SHD, structural heart disease (including ischemic heart disease); and SVT, supraventricular tachycardia.
2.4.1. Acute Treatment: Recommendations
Because patients with SVT account for approximately 50 000 emergency department visits each year,81 emergency physicians may be the first to evaluate patients whose tachycardia mechanism is unknown and to have the opportunity to diagnose the mechanism of arrhythmia. It is important to record a 12-lead ECG to differentiate tachycardia mechanisms according to whether the AV node is an obligate component (Section 2.3.2), because treatment that targets the AV node will not reliably terminate tachycardias that are not AV node dependent. Also, if the QRS duration is >120 ms, it is crucial to distinguish VT from SVT with aberrant conduction, pre-existing bundle-branch block, or pre-excitation (Table 4). In particular, the administration of verapamil or diltiazem for treatment of either VT or a pre-excited AF may lead to hemodynamic compromise or may accelerate the ventricular rate and lead to ventricular fibrillation.
| COR | LOE | Recommendations |
|---|---|---|
| I | B-R | 1. Vagal maneuvers are recommended for acute treatment in patients with regular SVT.82–84 |
| See Online Data Supplements 2 and 3. | For acute conversion of SVT, vagal maneuvers, including Valsalva and carotid sinus massage, can be performed quickly and should be the first-line intervention to terminate SVT. These maneuvers should be performed with the patient in the supine position. Vagal maneuvers typically will not be effective if the rhythm does not involve the AV node as a requisite component of a reentrant circuit. There is no "gold standard" for proper Valsalva maneuver technique but, in general, the patient raises intrathoracic pressure by bearing down against a closed glottis for 10 to 30 seconds, equivalent to at least 30 mm Hg to 40 mm Hg.82,84 Carotid massage is performed after absence of bruit has been confirmed by auscultation, by applying steady pressure over the right or left carotid sinus for 5 to 10 seconds.83,84 Another vagal maneuver based on the classic diving reflex consists of applying an ice-cold, wet towel to the face85; in a laboratory setting, facial immersion in water at 10°C (50°F) has proved effective in terminating tachycardia, as well.86 One study involving 148 patients with SVT demonstrated that Valsalva was more successful than carotid sinus massage, and switching from 1 technique to the other resulted in an overall success rate of 27.7%.82 The practice of applying pressure to the eyeball is potentially dangerous and has been abandoned. | |
| I | B-R | 2. Adenosine is recommended for acute treatment in patients with regular SVT.42,51,83,87–92 |
| See Online Data Supplements 2 and 3. | Adenosine has been shown in nonrandomized trials in the emergency department or prehospital setting to effectively terminate SVT that is due to either AVNRT or AVRT, with success rates ranging from 78% to 96%. Although patients may experience side effects, such as chest discomfort, shortness of breath, and flushing, serious adverse effects are rare because of the drug's very short half-life.93 Adenosine may also be useful diagnostically, to unmask atrial flutter or AT, but it is uncommon for adenosine to terminate these atrial arrhythmias.91 It should be administered via proximal IV as a rapid bolus infusion followed by a saline flush. Continuous ECG recording during adenosine administration may help diagnostically and can also distinguish drug failure due to failure to terminate the arrhythmias versus successful termination with immediate arrhythmia reinitiation. | |
| I | B-NR | 3. Synchronized cardioversion is recommended for acute treatment in patients with hemodynamically unstable SVT when vagal maneuvers or adenosine are ineffective or not feasible.94 |
| See Online Data Supplement 3. | Sinus rhythm must be promptly restored in patients with SVT who are hemodynamically unstable. The safety and effectiveness of cardioversion in the prehospital setting was analyzed in a cohort of patients with hemodynamically unstable SVT who had failed to convert with vagal maneuvers and intravenous pharmacological therapy, and cardioversion successfully restored sinus rhythm in all patients.94 The 2010 adult ACLS guideline75 advises synchronized cardioversion for any persistent SVT resulting in hypotension, acutely altered mental status, signs of shock, chest pain, or acute heart failure symptoms but recommends that adenosine be considered first if the tachycardia is regular and has a narrow QRS complex. | |
| I | B-NR | 4. Synchronized cardioversion is recommended for acute treatment in patients with hemodynamically stable SVT when pharmacological therapy is ineffective or contraindicated.87,95 |
| See Online Data Supplements 3 and 10. | Synchronized cardioversion is highly effective in terminating SVT (including AVRT and AVNRT), and when the patient is stable, this is performed after adequate sedation or anesthesia.94 Most stable patients with SVT respond to pharmacological therapy, with conversion success rates of 80% to 98% for agents such as verapamil, diltiazem, or adenosine. In some resistant cases, a second drug bolus or higher dose of initial drug agent might prove effective.87,96 Nevertheless, in rare instances, drugs may fail to successfully restore sinus rhythm, and cardioversion will be necessary. Synchronized cardioversion is inappropriate if the SVT is terminating and reinitiating spontaneously. | |
| IIa | B-R | 1. Intravenous diltiazem or verapamil can be effective for acute treatment in patients with hemodynamically stable SVT.87,89,92,97 |
| See Online Data Supplements 2 and 3. | Intravenous diltiazem and verapamil have been shown to terminate SVT in 64% to 98% of patients. These drugs should be used only in hemodynamically stable patients. A slow infusion of either drug up to 20 minutes may lessen the potential for hypotension.97 It is important to ensure that tachycardia is not due to VT or pre-excited AF because patients with these rhythms who are given diltiazem or verapamil may become hemodynamically unstable or may have accelerated ventricular rate, which may lead to ventricular fibrillation. These agents are especially useful in patients who cannot tolerate beta blockers or experience recurrence after conversion with adenosine. Diltiazem and verapamil are not appropriate for patients with suspected systolic heart failure. | |
| IIa | C-LD | 2. Intravenous beta blockers are reasonable for acute treatment in patients with hemodynamically stable SVT.96 |
| See Online Data Supplement 2. | Evidence for the effectiveness of beta blockers in terminating SVT is limited. In a trial that compared esmolol with diltiazem, diltiazem was more effective in terminating SVT.96 Nonetheless, beta blockers have an excellent safety profile, so it is reasonable to use intravenous beta blockers to attempt to terminate SVT in hemodynamically stable patients. | |
2.4.2. Ongoing Management: Recommendations
The recommendations and algorithm (Figure 9) for ongoing management, along with other recommendations and algorithms for specific SVTs that follow, are meant to include consideration of patient preferences and clinical judgment; this may include consideration of consultation with a cardiologist or clinical cardiac electrophyisiologist, as well as patient comfort with possible invasive diagnostic and therapeutic intervention. Recommendations for treatment options (including drug therapy, ablation, or observation) must be considered in the context of frequency and duration of the SVT, along with clinical manifestations, such as symptoms or adverse consequences (eg, development of cardiomyopathy).
| COR | LOE | Recommendations |
|---|---|---|
| I | B-R | 1. Oral beta blockers, diltiazem, or verapamil is useful for ongoing management in patients with symptomatic SVT who do not have ventricular pre-excitation during sinus rhythm.46,98,99 |
| See Online Data Supplement 2. | Although many patients prefer to undergo potentially curative therapy with ablation, given its high success rate, and although ablation may be mandatory therapy for patients in certain occupations (eg, pilots, bus drivers), patients may prefer not to undergo ablation or may not have access to a cardiac electrophysiologist. In these latter cases, pharmacological therapy with AV nodal blockers is an appropriate option for long-term prophylactic therapy. Pharmacological therapy with verapamil (dosage up to 480 mg/day) has been studied in RCTs, with reductions documented in SVT episode frequency and duration as recorded by Holter monitoring or subjective episode frequency recording in diaries.98 Evidence for beta blockers is limited. One small study randomized patients with SVT to digoxin (0.375 mg/day), propranolol (240 mg/day), or verapamil (480 mg/day), with 1 week of placebo washout between drug regimens.99 Reduction in the number of episodes and duration of SVT (ascertained by diary and weekly 24-h Holter) was similar among the treatment groups, and all 3 medications were well tolerated.99 | |
| I | B-NR | 2. EP study with the option of ablation is useful for the diagnosis and potential treatment of SVT.36,100–106 |
| See Online Data Supplement 2. | EP testing with the option of ablation is useful as first-line therapy for treatment of symptomatic SVT, as it provides the potential for definitive cure without the need for chronic pharmacological therapy. Large registry studies report high success rates for ablation of both AVNRT and AVRT, with low frequency of potentially serious complications (Table 8). | |
| I | C-LD | 3. Patients with SVT should be educated on how to perform vagal maneuvers for ongoing management of SVT.82 |
| See Online Data Supplement 2. | When properly performed, vagal maneuvers can terminate SVT, so patient education on this maneuver may help to avoid a more prolonged tachycardia episode and reduce the need to seek medical attention. Vagal maneuvers should be performed with the patient in the supine position. Patients can be taught to perform a Valsalva maneuver by forcefully exhaling against a closed airway for 10 to 30 seconds, equivalent to at least 30 mm Hg to 40 mm Hg.82,84 Another vagal maneuver based on the classic diving reflex consists of applying an ice-cold, wet towel to the face.85 | |
| IIa | B-R | 1. Flecainide or propafenone is reasonable for ongoing management in patients without structural heart disease or ischemic heart disease who have symptomatic SVT and are not candidates for, or prefer not to undergo, catheter ablation.45,46,107–112 |
| See Online Data Supplement 2. | Several RCTs have demonstrated the efficacy of daily therapy with propafenone (450 mg/day to 900 mg/day) or flecainide (100 mg/day to 300 mg/day) to prevent recurrences of SVT in symptomatic patients.45,46,107–112 In 1 RCT, the probability of 12 months of effective (defined as <2 attacks of arrhythmia) and safe treatment was 86% for propafenone and 93% for flecainide.109 However, flecainide and propafenone have a risk of proarrhythmia in patients with structural heart disease or ischemic heart disease, so these drugs are contraindicated in these patient groups.113 These drugs, though often effective, have potential side effects and as such should be reserved for patients for whom beta blockers, diltiazem, or verapamil are ineffective or cannot be prescribed. | |
| IIb | B-R | 1. Sotalol may be reasonable for ongoing management in patients with symptomatic SVT who are not candidates for, or prefer not to undergo, catheter ablation.114 |
| See Online Data Supplement 2. | Sotalol is a class III antiarrhythmic agent with beta-blocker properties. Unlike flecainide and propafenone, it can be used in patients with structural heart disease or ischemic heart disease. One study randomized patients with reentrant SVT (AVNRT or AVRT) or other atrial tachyarrhythmias (eg, AF, atrial flutter, AT) to sotalol at a dose of 80 mg or 160 mg twice daily or placebo and found significant reductions in recurrence risk, including for patients with reentrant SVT, with no proarrhythmic adverse effects.114 Because of the potential for proarrhythmia, sotalol should be reserved for patients who are not candidates for catheter ablation and for whom beta blockers, diltiazem, or verapamil are ineffective or cannot be prescribed. | |
| IIb | B-R | 2. Dofetilide may be reasonable for ongoing management in patients with symptomatic SVT who are not candidates for, or prefer not to undergo, catheter ablation and in whom beta blockers, diltiazem, flecainide, propafenone, or verapamil are ineffective or contraindicated.107 |
| See Online Data Supplement 2. | Dofetilide is a class III antiarrhythmic agent that, unlike sotalol, does not have beta-blocker properties. It may be reasonable in patients with structural heart disease or ischemic heart disease. In a trial of 122 patients randomized to dofetilide, propafenone, or placebo, the probability of remaining free of SVT after 6 months of treatment was 50% for dofetilide, 54% for propafenone, and 6% for placebo, with P<0.001 for either dofetilide or propafenone compared with placebo.107 Because of the potential for proarrhythmia, dofetilide should be reserved for patients who are not candidates for catheter ablation and for whom beta blockers, diltiazem, flecainide, verapamil, or propafenone are ineffective or cannot be prescribed. | |
| IIb | C-LD | 3. Oral amiodarone may be considered for ongoing management in patients with symptomatic SVT who are not candidates for, or prefer not to undergo, catheter ablation and in whom beta blockers, diltiazem, dofetilide, flecainide, propafenone, sotalol, or verapamil are ineffective or contraindicated.115 |
| See Online Data Supplement 2. | Evidence for amiodarone for the ongoing management of SVT is limited. The drug was evaluated in a small retrospective study and was found to be effective in suppressing AVNRT during outpatient follow-up.115 Amiodarone is a second-line agent for patients who are not able to take beta blockers, diltiazem, dofetilide, flecainide, propafenone, sotalol, or verapamil given the toxicity and side effects that may develop with long-term amiodarone therapy. | |
| IIb | C-LD | 4. Oral digoxin may be reasonable for ongoing management in patients with symptomatic SVT without pre-excitation who are not candidates for, or prefer not to undergo, catheter ablation.99 |
| See Online Data Supplement 2. | Evidence for the use of digoxin is limited. One small study randomized patients with SVT to digoxin (0.375 mg/day), propranolol (240 mg/day), and verapamil (480 mg/day), with 1 week of placebo washout between drug regimens.99 Overall, episodes and duration of SVT (ascertained by diary and weekly 24-h Holter) were similar, and all 3 medications were well tolerated.99 However, the dose of digoxin used was higher than that commonly used in clinical practice today, and in view of the risk of toxicity, digoxin should be reserved for patients who cannot take beta blockers, diltiazem, or verapamil or a class Ic agent (flecainide or propafenone) and must be used with caution in the presence of renal dysfunction. In some clinical studies, digoxin levels >1.2 ng/mL were associated with worse clinical outcomes, while levels <0.8 ng/mL were considered optimal; therefore, caution is advised.116 | |
2.5. Basic Principles of Electrophysiological Study, Mapping, and Ablation
2.5.1. Mapping With Multiple and Roving Electrodes
An invasive EP study permits the precise diagnosis of the underlying arrhythmia mechanism and localization of the site of origin and provides definitive treatment if coupled with catheter ablation. There are standards that define the equipment and training of personnel for optimal performance of EP study.141 EP studies involve placement of multielectrode catheters in the heart at ≥1 sites in the atria, ventricles, or coronary sinus. Pacing and programmed electrical stimulation may be performed with or without pharmacological provocation. Making a precise and correct diagnosis of the mechanism of SVT is the key to successful outcome, particularly when multiple arrhythmia mechanisms are possible; as such, appropriate diagnostic maneuvers should be performed before proceeding with ablation. By using diagnostic maneuvers during the EP study, the mechanism of SVT can be defined in most cases.80,142 Complications of diagnostic EP studies are rare but can be life threatening.143
Cardiac mapping is performed during EP studies to identify the site of origin of an arrhythmia or areas of critical conduction to allow targeting of ablation. Multiple techniques have been developed to characterize the temporal and spatial distribution of electrical activation.144 The simplest technique uses several multipole catheters plus a roving catheter that is sequentially positioned in different regions of interest and measures local activation time. Electroanatomic mapping systems and specialized multielectrode catheters, such as circular or multispline catheters, can map simultaneously from multiple sites and improve the speed and resolution of mapping.
2.5.2. Tools to Facilitate Ablation, Including 3-Dimensional Electroanatomic Mapping
Several tools have been developed to facilitate arrhythmia mapping and ablation, including electroanatomic 3-dimensional mapping and magnetic navigation. Potential benefits of these technologies include more precise definition or localization of arrhythmia mechanism, spatial display of catheters and arrhythmia activation, reduction in fluoroscopy exposure for the patient and staff, and shortened procedure times, particularly for complex arrhythmias or anatomy.145 Disadvantages include higher cost, as well as additional training, support, and procedure preparation time. Several studies have demonstrated the advantages of electroanatomic mapping, with success rates comparable to conventional approaches yet with significant reduction in fluoroscopy times.145–148
2.5.3. Mapping and Ablation With No or Minimal Radiation
Fluoroscopy has historically been the primary imaging modality used for EP studies. The use of ionizing radiation puts patient, operator, and laboratory staff at risk of the short- and long-term effects of radiation exposure. Attention to optimal fluoroscopic technique and adoption of radiation-reducing strategies can minimize radiation dose to the patient and operator. The current standard is to use the "as low as reasonably achievable" (ALARA) principle on the assumption that there is no threshold below which ionizing radiation is free from harmful biological effect. Alternative imaging systems, such as electroanatomic mapping and intracardiac echocardiography, have led to the ability to perform SVT ablation with no or minimal fluoroscopy, with success and complication rates similar to standard techniques.147,149–152 Radiation exposure may be further reduced by using robotic or magnetic navigation of catheters that use a 3-dimensional anatomic tracking system superimposed on traditional fluoroscopy imaging. A reduced- fluoroscopy approach is particularly important in pediatric patients and during pregnancy.153,154
2.5.4. Ablation Energy Sources
Radiofrequency current is the most commonly used energy source for SVT ablation.155 Cryoablation is used as an alternative to radiofrequency ablation to minimize injury to the AV node during ablation of specific arrhythmias, such as AVNRT, para-Hisian AT, and para-Hisian accessory pathways, particularly in specific patient populations, such as children and young adults. Selection of the energy source depends on operator experience, arrhythmia target location, and patient preference. Published trials, including a meta-analysis comparing radiofrequency ablation with cryoablation for treatment of AVNRT, have shown a higher rate of recurrence with cryoablation but lower risk of permanent AV nodal block.156–158 The rate of AVNRT recurrence with cryoablation depends on the size of the ablation electrode and the endpoint used.156,159 Ultimately, the choice of technology should be made on the basis of an informed discussion between the operator and the patient.
3. Sinus Tachyarrhythmias
In normal individuals, the sinus rate at rest is generally between 50 bpm and 90 bpm, reflecting vagal tone.160–163 Sinus tachycardia refers to the circumstance in which the sinus rate exceeds 100 bpm. Sinus tachycardia may be appropriate in response to physiological stimuli or other exogenous factors or may be inappropriate when the heart rate exceeds what would be expected for physical activity or other circumstances. On the ECG, the P wave is upright in leads I, II, and aVF and is biphasic in lead V1. As the sinus rate increases, activation arises from more superior aspects of the right atrium, resulting in a larger-amplitude P wave in the inferior leads.
3.1. Physiological Sinus Tachycardia
Sinus tachycardia is regarded as physiological when it is the result of appropriate autonomic influences, such as in the setting of physical activity or emotional responses. Physiological sinus tachycardia may result from pathological causes, including infection with fever, dehydration, anemia, heart failure, and hyperthyroidism, in addition to exogenous substances, including caffeine, drugs with a beta-agonist effect (eg, albuterol, salmeterol), and illicit stimulant drugs (eg, amphetamines, cocaine). In these cases, tachycardia is expected to resolve with correction of the underlying cause.
3.2. Inappropriate Sinus Tachycardia
Inappropriate sinus tachycardia (IST) is defined as sinus tachycardia that is unexplained by physiological demands at rest, with minimal exertion, or during recovery from exercise. Crucial to this definition is the presence of associated, sometimes debilitating, symptoms that include weakness, fatigue, lightheadedness, and uncomfortable sensations, such as heart racing. Patients with IST commonly show resting heart rates >100 bpm and average rates that are >90 bpm in a 24-hour period.160 The cause of IST is unclear, and mechanisms related to dysautonomia, neurohormonal dysregulation, and intrinsic sinus node hyperactivity have been proposed.
It is important to distinguish IST from secondary causes of tachycardia, including hyperthyroidism, anemia, dehydration, pain, and use of exogenous substances and drugs of abuse. Anxiety is also an important trigger, and patients with IST may have associated anxiety disorders.160 Structural heart disease, such as cardiomyopathies, must also be excluded, though the development of a cardiomyopathy secondary to sinus tachycardia is extremely rare.164,165 IST must also be distinguished from other forms of tachycardia, including AT arising from the superior aspect of the crista terminalis and sinus node reentrant tachycardia (Section 4). It is also important to distinguish IST from postural orthostatic tachycardia syndrome, although overlap may be present within an individual. Patients with postural orthostatic tachycardia syndrome have predominant symptoms related to a change in posture, and treatment to suppress the sinus rate may lead to severe orthostatic hypotension. Thus, IST is a diagnosis of exclusion.
3.2.1. Acute Treatment
There are no specific recommendations for acute treatment of IST.
3.2.2. Ongoing Management: Recommendations
Because the prognosis of IST is generally benign, treatment is for symptom reduction and may not be necessary. Treatment of IST is difficult, and it should be recognized that lowering the heart rate may not alleviate symptoms. Therapy with beta blockers or calcium channel blockers is often ineffective or not well tolerated because of cardiovascular side effects, such as hypotension. Exercise training may be of benefit, but the benefit is unproven.
Ivabradine is an inhibitor of the "I-funny" or "If" channel, which is responsible for normal automaticity of the sinus node; therefore, ivabradine reduces the sinus node pacemaker activity, which results in slowing of the heart rate. On the basis of the results of 2 large, randomized, placebo-controlled trials, this drug was recently approved by the FDA for use in patients with systolic heart failure. In the BEAUTIFUL (Morbidity-Mortality Evaluation of the If Inhibitor Ivabradine in Patients With Coronary Disease and Left-Ventricular Dysfunction) study,166 10 917 patients with coronary disease and a left ventricular ejection fraction <40% were randomized to ivabradine or placebo. In the SHIFT (Systolic Heart Failure Treatment With the If Inhibitor Ivabradine) trial,167 6558 patients with a left ventricular ejection fraction ≤35% were randomized to ivabradine or placebo. In both of these trials, therapy with ivabradine resulted in additional heart rate reductions of 6 to 8 bpm and proved to be generally safe. The drug has no other hemodynamic effects aside from lowering the heart rate. As such, it has been investigated for use to reduce the sinus rate and improve symptoms related to IST.168–176
Radiofrequency ablation to modify the sinus node can reduce the sinus rate, with acute procedural success rates reported in the range of 76% to 100% in nonrandomized cohorts.177–183 Ablation is typically performed with 3-dimensional electroanatomic or noncontact mapping techniques targeting sites of early activation with isoproterenol infusion, with or without use of intracardiac ultrasound–guided mapping to image the crista terminalis. Nonetheless, symptoms commonly recur after several months, with IST recurrence in up to 27% and overall symptomatic recurrence (IST or non-IST AT) in 45% of patients.177,179,180,182 Complications can be significant and may include symptomatic sinus or junctional bradycardia necessitating pacemaker placement, phrenic nerve injury with paralysis of the right hemidiaphragm, and significant facial and upper-extremity swelling caused by narrowing of the superior vena cava/RA junction, which may rarely result in superior vena cava syndrome. In view of the modest benefit of this procedure and its potential for significant harm, sinus node modification should be considered only for patients who are highly symptomatic and cannot be adequately treated by medication, and then only after informing the patient that the risks may outweigh the benefits of ablation. Even more aggressive surgical methods to ablate or denervate the sinus node have been described, further highlighting the risks that highly symptomatic patients are willing to accept to find relief.184 Effective patient communication is key for these patients.
| COR | LOE | Recommendations |
|---|---|---|
| I | C-LD | 1. Evaluation for and treatment of reversible causes are recommended in patients with suspected IST.160,185 |
| See Online Data Supplements 4 and 5. | It is important to distinguish IST from physiological sinus tachycardia or focal AT from the high right atrium, which can have P-wave morphology similar to the sinus P wave. A careful history and physical examination, with further laboratory and imaging studies, are necessary to determine reversible causes of tachycardia, such as exogenous substances and drugs, infection, anemia, and hyperthyroidism. A focal AT would have sudden onset and termination, which would not be the case for IST. | |
| IIa | B-R | 1. Ivabradine is reasonable for ongoing management in patients with symptomatic IST.168–176 |
| See Online Data Supplements 4 and 5. | In 1 small randomized crossover trial,168 ivabradine given at a dosage of 2.5 to 7.5 mg twice daily significantly reduced daytime heart rate from 98.4±11.2 at baseline to 84.7±9.0, compared with 98.6±11.1 on placebo (P<0.001), and improved exercise tolerance and symptoms in patients with IST. Similar findings have been observed in several additional nonrandomized observational studies.169–176 Furthermore, a significant number of patients in these studies reported complete resolution of symptoms, with persistent clinical benefit observed in some even after discontinuing the drug. In 1 observational study, ivabradine was more effective than metoprolol in reduction of the heart rate and amelioration of symptoms.170 The drug is well tolerated, with an excellent safety profile demonstrated in 2 large RCTs in patients with heart failure.166,167 Ivabradine can cause phosphenes, an enhanced brightness in a portion of the visual field; this side effect, which is usually transient, was reported in 3% of patients taking the drug in the SHIFT trial.167 | |
| IIb | C-LD | 1. Beta blockers may be considered for ongoing management in patients with symptomatic IST.170,172 |
| See Online Data Supplement 4 and 5. | Beta blockers are modestly effective in lowering the heart rate and improving symptoms that are due to IST. In a small nonrandomized observational cohort, metoprolol succinate titrated to a target of 95 mg daily lowered the heart rate over 4 weeks from a baseline.172 In a small nonrandomized study comparing metoprolol with ivabradine, both agents reduced heart rate compared with baseline and improved exercise capacity.170 Although effectiveness of beta blockers is modest and hypotension may limit dose, the overall safety of beta blockers warrants their use for treatment of symptomatic patients. | |
| IIb | C-LD | 2. The combination of beta blockers and ivabradine may be considered for ongoing management in patients with IST.172 |
| See Online Data Supplement 4 and 5. | Some patients with IST may have particularly refractory symptoms, and single-drug efficacy may be limited. In a small observational study, the addition of ivabradine (7.5 mg twice daily) to metoprolol succinate (95 mg daily) reduced the heart rate from baseline to a greater extent than did metoprolol alone.172 On combination therapy, symptoms related to IST were resolved in all patients, and the combined therapy was well tolerated. In the SHIFT and BEAUTIFUL studies, the majority of patients were taking the combination of ivabradine and a beta blocker, which was well tolerated.166,167 Nevertheless, patients who are considered for combination therapy should be monitored closely for the possibility of excess bradycardia. Ivabradine can cause phosphenes, an enhanced brightness in a portion of the visual field; this side effect was reported in 3% of patients taking the drug in the SHIFT trial.167 | |
4. Nonsinus Focal Atrial Tachycardia and MAT
See Figure 10 for the algorithm for acute treatment of suspected focal atrial tachycardia (AT) and Figure 11 for the algorithm for ongoing management of focal AT.

- Download figure
- Download PowerPoint
Figure 10. Acute treatment of suspected focal atrial tachycardia. Colors correspond to Class of Recommendation in Table 1; drugs listed alphabetically. *For rhythms that break or recur spontaneously, synchronized cardioversion is not appropriate. IV indicates intravenous.

- Download figure
- Download PowerPoint
Figure 11. Ongoing management of focal atrial tachycardia. Colors correspond to Class of Recommendation in Table 1; drugs listed alphabetically. Pt indicates patient; and SHD, structural heart disease (including ischemic heart disease).
4.1. Focal Atrial Tachycardia
Focal AT is characterized as a fast rhythm from a discrete origin, discharging at a rate that is generally regular, and conducting in a centrifugal manner throughout the atrial tissue. Focal AT represents approximately 3% to 17% of the patients referred for SVT ablation.49,122,186 The demographics of focal AT in the adult population will continue to change as SVTs are increasingly ablated at a younger age.
Focal AT can be sustained or nonsustained. The atrial rate during focal AT is usually between 100 and 250 bpm.186 Presence and severity of symptoms during focal AT are variable among patients. Focal AT in the adult population is usually associated with a benign prognosis, although AT-mediated cardiomyopathy has been reported in up to 10% of patients referred for ablation of incessant SVT.187,188 Nonsustained focal AT is common and often does not require treatment.
The diagnosis of focal AT is suspected when the ECG criteria are met (Section 2). Algorithms have been developed to estimate the origin of the focal AT from the P-wave morphology recorded on a standard 12-lead ECG.189,190 In general, a positive P wave in lead V1 and negative P waves in leads I and aVL are correlated to ATs arising from the left atrium. Positive P waves in leads II, III, and aVF suggest that the origin of AT is from the cranial portion of either atria. Shorter P-wave duration is correlated to AT arising from the paraseptal tissue versus the right or left atrial free wall.191 The precise location of the focal AT is ultimately confirmed by mapping during EP studies when successful ablation is achieved.123–127,192–196 Focal AT has been localized to the crista terminalis, right or left atrial free wall or appendage, tricuspid or mitral annulus, paraseptal or paranodal areas, pulmonary veins, coronary sinus, and coronary cusps, but it originates more frequently from the right atrium than from the left atrium.197,198
The underlying mechanism of focal AT can be automatic, triggered activity, or microreentry, but methods to distinguish the mechanism through pharmacological testing or EP study are of modest value because of limited sensitivity and specificity.123,199,200 An automatic AT can be transiently suppressed by adenosine or by overdrive pacing and may be terminated by beta blockers, diltiazem, or verapamil. Whereas a triggered AT can be terminated by adenosine or overdrive pacing, its response to beta blockers, diltiazem, or verapamil may be variable. A microreentrant AT can be induced and terminated by programmed stimulation, but its response to adenosine, beta blockers, diltiazem, or verapamil may depend on the location of the microreentrant circuit; the tachycardia can be terminated by these drugs if the microreentrant circuit involves tissue around the sinus node, whereas microreentrant ATs from other locations generally will not be terminated by these drugs.
Sinus node reentrant tachycardia is an uncommon type of focal AT that involves a microreentrant circuit in the region of the sinoatrial node, causing a P-wave morphology that is identical to that of sinus tachycardia (although this is not sinus tachycardia). Characteristics that distinguish sinus node reentry from sinus tachycardia are an abrupt onset and termination and often a longer RP interval than that observed during normal sinus rhythm. Sinus node reentry is characterized by paroxysmal episodes of tachycardia, generally 100 bpm to 150 bpm.201–203 Confirmation of the reentrant mechanism requires an EP study. Induction of sinus node reentrant tachycardia during programmed stimulation, demonstration of entrainment, and localization of the tachycardia origin in the region of the sinus node are necessary to confirm the diagnosis.
4.1.1. Acute Treatment: Recommendations
RCTs of drug therapy for comparative effectiveness in patients with focal AT in the acute setting are not available. Many of the clinical outcomes are reported from small observational studies that included infants or pediatric patients.204,205 The design or execution of these studies is frequently suboptimal because of the poorly defined inclusion criteria or variable clinical settings. Several studies included a mix of patients with congenital or postoperative AT, and some of these patients likely had macroreentrant AT. In many reports, the response to intravenous drug therapy was evaluated by EP study rather than in the clinical environment.123,200,204–207 In the clinical setting, if the diagnosis is uncertain, vagal maneuvers may be attempted to better identify the mechanism of SVT.
Digoxin has not been well studied for focal AT. Intravenous class Ic drugs (eg, flecainide, propafenone) may be moderately effective in treating focal AT in the acute setting, as reported in earlier, small observational studies, although intravenous forms of IC drugs are not available in the United States. In patients with an implanted cardiac pacing device, it may be possible to perform overdrive pacing through the device, although close monitoring is required to prevent any significant adverse effect, such as pacing-induced AF or other atrial arrhythmias. Equipment should be available to provide support for cardioversion of AF if needed.
| COR | LOE | Recommendations |
|---|---|---|
| I | C-LD | 1. Intravenous beta blockers, diltiazem, or verapamil is useful for acute treatment in hemodynamically stable patients with focal AT.123,204,205,207 |
| See Online Data Supplement 6. | Intravenous beta blockers, diltiazem, or verapamil is recommended to treat focal AT. During EP study, propranolol or verapamil is moderately effective in either terminating the focal AT or slowing the ventricular rate in approximately 30% to 50% of the patients.204,205 Although these agents are relatively safe, close monitoring is recommended during intravenous drug therapy to evaluate for hypotension or bradycardia. | |
| I | C-LD | 2. Synchronized cardioversion is recommended for acute treatment in patients with hemodynamically unstable focal AT.94,208 |
| See Online Data Supplement 6. | Although minimum data are available on cardioversion of focal AT, synchronized cardioversion is a consideration in patients with drug-resistant arrhythmias associated with signs and symptoms of compromised hemodynamics. Termination of tachycardia is expected when a focal AT is of a microreentrant mechanism. Response of a triggered focal AT to cardioversion can be variable, whereas electrical cardioversion is not likely to be effective in focal AT with an automatic mechanism. In this latter case, antiarrhythmic drug therapy is usually required. | |
| IIa | B-NR | 1. Adenosine can be useful in the acute setting to either restore sinus rhythm or diagnose the tachycardia mechanism in patients with suspected focal AT.123,200,207 |
| See Online Data Supplement 6. | Adenosine is usually effective in terminating focal AT of a triggered mechanism but is not expected to be effective in reentrant focal AT.207 Transient suppression can be observed in automatic focal AT. The observation of transient AV block with persistent AT can be helpful in making the diagnosis and differentiating focal AT from AVNRT and AVRT. | |
| IIb | C-LD | 1. Intravenous amiodarone may be reasonable in the acute setting to either restore sinus rhythm or slow the ventricular rate in hemodynamically stable patients with focal AT.205,206 |
| See Online Data Supplement 6. | The therapeutic effect of intravenous amiodarone in the acute setting is likely mediated via blockade of the beta receptors or calcium channels. Amiodarone may be preferred in patients with reduced ventricular function or with a history of heart failure. | |
| IIb | C-LD | 2. Ibutilide may be reasonable in the acute setting to restore sinus rhythm in hemodynamically stable patients with focal AT.205,206 |
| See Online Data Supplement 6. | The effectiveness of ibutilide for treatment of focal AT is unclear. In 1 study, intravenous ibutilide terminated AT of single atrial morphology in 19 of 39 patients (38.8%), but the proportions of patients with focal AT and macroreentrant AT were not differentiated in this study cohort.206 | |
4.1.2. Ongoing Management: Recommendations
| COR | LOE | Recommendations |
|---|---|---|
| I | B-NR | 1. Catheter ablation is recommended in patients with symptomatic focal AT as an alternative to pharmacological therapy.122–126,188,191–196,206 |
| See Online Data Supplement 6. | A large number of nonrandomized cohort studies on focal AT ablation have accumulated in the past 2 decades. In a 2012 ablation registry provided by 74 voluntary medical centers in Spain, AT was found in 333 of 11 042 of the ablation procedures performed.122 In experienced centers, when the AT can be induced in the laboratory, acute success rates above 90% to 95% have consistently been reported, with a complication rate of <1% to 2%.122,123,125,196 See Table 8 for a summary of ablation efficacy, complications, and rate of recurrence.Although uncommon, focal AT-mediated cardiomyopathy should be recognized in patients presenting with heart failure, reduced ventricular function, and persistent tachycardia. In a case–control study of patients with AT, 10% of patients had evidence of cardiomyopathy.125 The tachycardia in patients with cardiomyopathy was incessant and slower than in the patients without cardiomyopathy (cycle lengths 502 ms and 402 ms, respectively). Normal ejection fraction was restored in 97% of patients after successful ablation.188 | |
| IIa | C-LD | 1. Oral beta blockers, diltiazem, or verapamil are reasonable for ongoing management in patients with symptomatic focal AT.123,204,205 |
| See Online Data Supplement 6. | Data on long-term drug therapy of focal AT are limited to observational studies, and some studies did not provide clear inclusion criteria, so results for AT were combined with those for other mechanisms of SVT. Nevertheless, these drugs are moderately effective, with a low incidence of significant adverse effects.123,204,205,209–214 | |
| IIa | C-LD | 2. Flecainide or propafenone can be effective for ongoing management in patients without structural heart disease or ischemic heart disease who have focal AT.209–213 |
| See Online Data Supplement 6. | Small case series studies reported that focal AT suppression was achieved with flecainide in most patients.209,210 In infants and children, propafenone is moderately effective in focal AT suppression during follow-up.213 Flecainide and propafenone are generally tolerated by patients with focal AT. Combinations of a class Ic drug with a beta blocker, diltiazem, or verapamil may improve overall efficacy rates. | |
| IIb | C-LD | 1. Oral sotalol or amiodarone may be reasonable for ongoing management in patients with focal AT.188,211,215–219 |
| See Online Data Supplement 6. | Several studies reported moderate efficacies of oral sotalol or amiodarone in maintaining sinus rhythm in long-term treatment in children.188,211,215–219 Although most reports are in children, limited data suggest similar efficacy in adults.218 Because of the risk of proarrhythmia and other complications, before use of these drugs, a balance between anticipated benefit of focal AT suppression and potential adverse effects of these drugs should be carefully considered. | |
4.2. Multifocal Atrial Tachycardia
MAT is defined as a rapid, irregular rhythm with at least 3 distinct morphologies of P waves on the surface ECG. It may be difficult to distinguish MAT from AF on physical examination or even on a single ECG tracing, so a 12-lead ECG is indicated to confirm the diagnosis. On the ECG, the atrial rate is >100 bpm (or >90 bpm, as defined in at least 1 report220). Unlike AF, there is a distinct isoelectric period between P waves. The P-P, P-R, and R-R intervals are variable. The mechanism of MAT is not well established. Although it is assumed that the variability of P-wave morphology implies a multifocal origin, there are very few mapping studies of MAT.221 Similarly, the variability of the P-R interval may relate to decremental conduction through the AV node, as opposed to the origin of the P wave. Occasional responsiveness to verapamil suggests a triggered mechanism, but data are limited.222
MAT is commonly associated with underlying conditions, including pulmonary disease, pulmonary hypertension, coronary disease, and valvular heart disease,223 as well as hypomagnesemia and theophylline therapy.224 The first-line treatment is management of the underlying condition. Intravenous magnesium may also be helpful in patients with normal magnesium levels.225 Antiarrhythmic medications in general are not helpful in suppression of multifocal AT.226 Management often involves slowing conduction at the AV nodal level to control heart rate. Verapamil has been shown to have some efficacy in patients with MAT who do not have ventricular dysfunction, sinus node dysfunction, or AV block227,228; although diltiazem has not been studied, it may provide a class effect with similar mechanism to verapamil. Beta blockers can be used with caution to treat MAT in the absence of respiratory decompensation, sinus node dysfunction, or AV block.229,230 Amiodarone has been reported to be useful in 1 report.231 Cardioversion is not useful in MAT.223
4.2.1. Acute Treatment: Recommendation
| COR | LOE | Recommendation |
|---|---|---|
| IIa | C-LD | 1. Intravenous metoprolol229 or verapamil232,233 can be useful for acute treatment in patients with MAT. |
| See Online Data Supplement 7. | The mechanism of MAT can involve triggered activity, and treatment with intravenous verapamil can terminate the arrhythmia with moderate success. In 1 small study, intravenous verapamil converted MAT in 8 of 16 patients treated.233 Alternatively, intravenous verapamil may acutely slow the ventricular response to MAT. The major potential side effect is hypotension.233 The relatively cardioselective beta blocker metoprolol can also work by slowing the ventricular rate in MAT. Beta blockers are typically avoided in patients with severe underlying pulmonary disease, particularly those with bronchospasm; both beta blockers and verapamil are typically avoided in the presence of acute decompensated heart failure and/or hemodynamic instability. | |
4.2.2. Ongoing Management: Recommendations
| COR | LOE | Recommendations |
|---|---|---|
| IIa | B-NR | 1. Oral verapamil (Level of Evidence: B-NR ) or diltiazem (Level of Evidence: C-LD) is reasonable for ongoing management in patients with recurrent symptomatic MAT.227,230 |
| C-LD | ||
| See Online Data Supplement 8. | Long-term management of MAT frequently involves slowing of the ventricular response because arrhythmia termination is often not achievable. Verapamil has the advantage of not exacerbating pulmonary disease. Although it would be expected for diltiazem to have a similar effect, data on its use in patients with MAT are lacking. These drugs should not be used in patients with severe conduction abnormalities or sinus node dysfunction because those conditions can be exacerbated with these agents. | |
| IIa | C-LD | 2. Metoprolol is reasonable for ongoing management in patients with recurrent symptomatic MAT.226,229,230 |
| See Online Data Supplements 7 and 8. | Beta blockers are typically avoided in the presence of acute decompensated heart failure or in patients with severe (particularly bronchospastic) pulmonary disease. However, metoprolol has been used in small studies in patients with serious pulmonary disease after correction of hypoxia or other signs of acute decompensation. In these studies, intravenous or oral metoprolol resulted in conversion to sinus rhythm or achieved rate control, and oral metoprolol was used for maintenance therapy.226,230 Beta blockers are generally avoided in patients with severe conduction abnormalities or sinus node dysfunction. | |
5. Atrioventricular Nodal Reentrant Tachycardia
See Figure 12 for the algorithm for acute treatment of AVNRT and Figure 13 for the algorithm for ongoing management of AVNRT.

- Download figure
- Download PowerPoint
Figure 12. Acute treatment of AVNRT. Colors correspond to Class of Recommendation in Table 1; drugs listed alphabetically. *For rhythms that break or recur spontaneously, synchronized cardioversion is not appropriate. AVNRT indicates atrioventricular nodal reentrant tachycardia; and IV, intravenous.

- Download figure
- Download PowerPoint
Figure 13. Ongoing management of AVNRT. Colors correspond to Class of Recommendation in Table 1; drugs listed alphabetically. AVNRT indicates atrioventricular nodal reentrant tachycardia; pt, patient; and SHD, structural heart disease (including ischemic heart disease).
AVNRT is the most common SVT. It is usually seen in young adults without structural heart disease or ischemic heart disease, and >60% of cases are observed in women.49 The ventricular rate is often 180 bpm to 200 bpm but ranges from 110 bpm to >250 bpm (and in rare cases, the rate can be <100 bpm).54 The anatomic substrate of AVNRT is dual AV nodal physiology (Table 3).
AVNRT is often well tolerated and is rarely life threatening. Patients will typically present with the sudden onset of palpitations and possibly with shortness of breath, dizziness, and neck pulsations. Syncope is a rare manifestation of AVNRT. AVNRT may occur spontaneously or on provocation with exertion, coffee, tea, or alcohol.
5.1. Acute Treatment: Recommendations
| COR | LOE | Recommendations |
|---|---|---|
| I | B-R | 1. Vagal maneuvers are recommended for acute treatment in patients with AVNRT.82–84,234,235 |
| See Online Data Supplement 10. | For acute conversion of AVNRT, vagal maneuvers, including Valsalva and carotid sinus massage, can be performed quickly and should be the first-line intervention to terminate SVT. These maneuvers should be performed with the patient in the supine position. There is no "gold standard" for proper Valsalva maneuver technique, but in general, the patient raises intrathoracic pressure by bearing down against a closed glottis for 10 to 30 seconds, equivalent to at least 30 mm Hg to 40 mm Hg.82,84 Carotid massage is performed after absence of bruit has been confirmed by auscultation, by applying steady pressure over the right or left carotid sinus for 5 to10 seconds.83,84 Another vagal maneuver based on the classic diving reflex consists of applying an ice-cold, wet towel to the face85; in a laboratory setting, facial immersion in water at 10°C (50°F) has proved effective in terminating tachycardia, as well.86 One study involving 148 patients with SVT demonstrated that Valsalva was more successful than carotid sinus massage, and switching from 1 technique to the other resulted in an overall success rate of 27.7%.82 The practice of applying pressure to the eyeball is potentially dangerous and has been abandoned. | |
| I | B-R | 2. Adenosine is recommended for acute treatment in patients with AVNRT.42,51,91,236 |
| See Online Data Supplements 9 and 10. | Adenosine can be considered as both a therapeutic and diagnostic agent in narrow-complex tachyarrhythmias. It will acutely terminate AVNRT in approximately 95% of patients and will unmask atrial activity in arrhythmias, such as atrial flutter or AT.91,236 | |
| I | B-NR | 3. Synchronized cardioversion should be performed for acute treatment in hemodynamically unstable patients with AVNRT when adenosine and vagal maneuvers do not terminate the tachycardia or are not feasible.94,208 |
| See Online Data Supplement 10. | Sinus rhythm must be promptly restored in patients with AVNRT who are hemodynamically unstable. The safety and effectiveness of cardioversion has been proven in patients with hemodynamically unstable SVT who had failed to convert with vagal maneuvers and intravenous pharmacological therapy.94 | |
| I | B-NR | 4. Synchronized cardioversion is recommended for acute treatment in hemodynamically stable patients with AVNRT when pharmacological therapy does not terminate the tachycardia or is contraindicated.87,95 |
| See Online Data Supplements 3 and 9. | Synchronized cardioversion is highly effective in terminating SVT (including AVRT and AVNRT).94 Most stable patients with SVT respond to pharmacological therapy, with success rates of 80% to 98% for agents such as verapamil, diltiazem, or adenosine. In some resistant cases, a second drug bolus or higher dose of initial drug agent is often effective.87,96 Nevertheless, in rare instances, drugs may fail to successfully restore sinus rhythm, necessitating synchronized cardioversion. | |
| IIa | B-R | 1. Intravenous beta blockers, diltiazem, or verapamil are reasonable for acute treatment in hemodynamically stable patients with AVNRT.96,237–240 |
| See Online Data Supplement 10. | Intravenous diltiazem and verapamil are particularly effective in converting AVNRT to sinus rhythm. These drugs should be used only in hemodynamically stable patients. It is important to ensure the absence of VT or pre-excited AF, because patients with these rhythms may become hemodynamically unstable and develop ventricular fibrillation if administered diltiazem or verapamil. Diltiazem or verapamil should also be avoided in patients with suspected systolic heart failure. Evidence for the effectiveness of beta blockers to terminate AVNRT is limited. In a trial that compared esmolol with diltiazem, diltiazem was more effective in terminating SVT.237 Nonetheless, beta blockers have an excellent safety profile, so it is reasonable to use them to attempt to terminate SVT in hemodynamically stable patients. | |
| IIb | C-LD | 1. Oral beta blockers, diltiazem, or verapamil may be reasonable for acute treatment in hemodynamically stable patients with AVNRT.241,242 |
| See Online Data Supplement 9. | Overall, there are no data specifically studying the effect of oral beta-blocker monotherapy for the acute termination of AVNRT. However, 2 studies have demonstrated success with the combination of oral diltiazem and propranolol to terminate AVNRT or AVRT.241,242 Oral beta blockers have an excellent safety profile, and administration (particularly in patients without intravenous access) can be performed in conjunction with vagal maneuvers. | |
| IIb | C-LD | 2. Intravenous amiodarone may be considered for acute treatment in hemodynamically stable patients with AVNRT when other therapies are ineffective or contraindicated.115 |
| See Online Data Supplement 10. | In a small cohort study, intravenous amiodarone was effective in terminating AVNRT.115 Long-term toxicity is not seen with intravenous amiodarone if given for a short period of time. | |
5.2. Ongoing Management: Recommendations
| COR | LOE | Recommendations |
|---|---|---|
| I | B-R | 1. Oral verapamil or diltiazem is recommended for ongoing management in patients with AVNRT who are not candidates for, or prefer not to undergo, catheter ablation.98,99,243,244 |
| See Online Data Supplements 9 and 10. | Both diltiazem and verapamil are well-tolerated and effective pharmacological alternatives to ablation for the ongoing treatment of patients with AVNRT.98,243 When therapy with these agents is initiated, attention should be directed toward avoiding the potential for bradyarrhythmias and hypotension. Diltiazem and verapamil should also be avoided in patients with systolic heart failure. | |
| I | B-NR | 2. Catheter ablation of the slow pathway is recommended in patients with AVNRT.36,100–106,245–249 |
| See Online Data Supplements 9 and 10. | Catheter ablation of AVNRT is regarded as first-line therapy for treatment of symptomatic AVNRT. It is potentially curative, and chronic pharmacological therapy is usually not needed after the procedure. Slow-pathway ablation (also called modification) is the preferred target during ablation of AVNRT.Large registry studies report the success rates of slow-pathway ablation to be >95%, with a <1% risk of AV block (Table 8).36,100–102,246–248 Cryoablation of AVNRT is an alternative to radiofrequency ablation. Recent systematic reviews and trials randomizing patients to radiofrequency ablation versus cryoablation suggest an equivalent acute success rate, with a lower rate of AV block but a higher rate of recurrence during long-term follow-up.156 | |
| I | B-R | 3. Oral beta blockers are recommended for ongoing management in patients with AVNRT who are not candidates for, or prefer not to undergo, catheter ablation.99 |
| See Online Data Supplement 9. | Evidence for oral beta blockers is limited. One small study randomized patients with AVNRT and AVRT to digoxin (0.375 mg/day), propranolol (240 mg/day), or verapamil (480 mg/day), with 1 week of placebo washout between drug regimens.99 Episodes and duration of tachyarrhythmia (ascertained by diary and weekly 24-h Holter) were similar among the treatment groups, and all 3 medications were well tolerated. | |
| IIa | B-R | 1. Flecainide or propafenone is reasonable for ongoing management in patients without structural heart disease or ischemic heart disease who have AVNRT and are not candidates for, or prefer not to undergo, catheter ablation and in whom beta blockers, diltiazem, or verapamil are ineffective or contraindicated.45,46,107–112,114,241,242,250,251 |
| See Online Data Supplements 9 and 10. | In 1 RCT, the probability of 12 months of effective (defined as <2 attacks of arrhythmia) and safe treatment was 86% for propafenone and 93% for flecainide.109 Flecainide and propafenone have a risk of proarrhythmia in patients with structural heart disease or ischemic heart disease and are contraindicated in these patient groups. In 1 nonrandomized study, flecainide was evaluated as "pill-in-the-pocket" therapy along with diltiazem or propranolol.241,242 However, all patients were screened with EP studies, and only 5 patients were discharged on flecainide. As such, the merit of flecainide as "pill in the pocket" for outpatient therapy for AVNRT remains unclear. | |
| IIa | B-NR | 2. Clinical follow-up without pharmacological therapy or ablation is reasonable for ongoing management in minimally symptomatic patients with AVNRT.244 |
| See Online Data Supplement 10. | In a prospective study of 93 adult patients with AVNRT who were followed for approximately 15 years, nearly half of minimally symptomatic patients who received no therapy (versus ablation or antiarrhythmic agents) improved over time and became asymptomatic.244 Patients with a confirmed or suspected diagnosis of AVNRT who choose not to undergo catheter ablation or take medications should be educated about when to seek medical attention and be taught how to perform vagal maneuvers. | |
| IIb | B-R | 1. Oral sotalol or dofetilide may be reasonable for ongoing management in patients with AVNRT who are not candidates for, or prefer not to undergo, catheter ablation.107,114 |
| See Online Data Supplement 9. | Unlike flecainide and propafenone, sotalol and dofetilide can be used in patients with structural heart disease or coronary artery disease.113 Given the potential for significant QT prolongation and torsades de pointes, inpatient monitoring with serial ECGs is generally performed when these agents are initiated. Generally, these agents are reserved for patients who are unresponsive to, or are not candidates for, beta blockers, diltiazem, flecainide, propafenone, or verapamil. | |
| IIb | B-R | 2. Oral digoxin or amiodarone may be reasonable for ongoing treatment of AVNRT in patients who are not candidates for, or prefer not to undergo, catheter ablation.99,115 |
| See Online Data Supplements 9 and 10. | One small study randomized patients with unspecified PSVT to digoxin (0.375 mg/day), propranolol (240 mg/day), or verapamil (480 mg/day), with 1 week of placebo washout.99 Episodes and duration of PSVT (ascertained by diary and weekly 24-h Holter) were largely similar, and all 3 medications were well tolerated.99 However, the dose of digoxin used was higher than that commonly used in clinical practice today. Amiodarone is effective in suppressing AVNRT during outpatient follow-up.115 Given the potential adverse effects of digoxin and amiodarone, these agents are generally reserved as third-line therapy for patients who are unresponsive to, or are not candidates for, beta blockers, diltiazem, verapamil, flecainide, or propafenone. | |
| IIb | C-LD | 3. Self-administered ("pill-in-the-pocket") acute doses of oral beta blockers, diltiazem, or verapamil may be reasonable for ongoing management in patients with infrequent, well-tolerated episodes of AVNRT.241,242 |
| See Online Data Supplement 9. | Two studies have demonstrated success with the combination of diltiazem and propranolol as a "pill-in-the-pocket" approach to acutely terminate PSVT caused by AVNRT, but the overall safety of self-administration of these medications remains unclear because episodes of syncope were observed.241,242 If oral therapy with empiric beta blockers, diltiazem, or verapamil fails to terminate the tachyarrhythmia, patients should seek medical attention. | |
6. Manifest and Concealed Accessory Pathways
Accessory pathways can be manifest or concealed; can conduct in the anterograde direction, retrograde direction, or both; and can be associated with several different supraventricular arrhythmias. Some anterograde pathways may place patients at risk of SCD. Typically, pathways directly connect the atrium and ventricle, bypassing the normal conduction through the AV node and His Purkinje system. The pathways are considered manifest if they conduct in the anterograde direction, demonstrating pre-excitation with a delta wave on the ECG. Manifest pathways occur in 0.1% to 0.3% of the population and may conduct in both the anterograde and retrograde directions or, less commonly, only in the anterograde direction.252 Concealed pathways conduct only in the retrograde direction and therefore do not cause pre-excitation on the standard 12-lead ECG.
The most common tachycardia associated with an accessory pathway is orthodromic AVRT, with a circuit that uses the AV node and His Purkinje system in the anterograde direction, followed by conduction through the ventricle, retrograde conduction over the accessory pathway, and completion of the circuit by conduction through the atrium back into the AV node. Orthodromic AVRT accounts for approximately 90% to 95% of AVRT episodes in patients with a manifest accessory pathway. Pre-excited AVRT, including antidromic AVRT, accounts for 5% of the AVRT episodes in patients with a manifest pathway and involves conduction from the atrium to the ventricle via the accessory pathway, causing a pre-excited QRS complex. This is called antidromic AVRT tachycardia when the return reentrant conduction occurs retrogradely via the AV node. In rare cases of pre-excited AVRT, the return conduction occurs via a second accessory AV pathway. AF can occur in patients with accessory pathways, which may result in extremely rapid conduction to the ventricle over a manifest pathway, which increases the risk of inducing ventricular fibrillation and SCD. Other SVTs, such as AVNRT, AT, and atrial flutter, can also conduct rapidly over a manifest accessory pathway; in these instances, the pathway is considered a "bystander" because it is not part of the tachycardia circuit. Most accessory pathways have conduction properties similar to the myocardium and do not demonstrate decremental conduction. A unique form of AVRT involves a concealed accessory pathway, usually located in the posteroseptal region, with retrograde decremental conduction properties resulting in a form of orthodromic reentrant tachycardia termed PJRT. This tachycardia has deeply inverted retrograde P waves in leads II, III, and aVF, with a long RP interval due to the location and decremental conduction properties of the accessory pathway (Figure 6). The incessant nature of PJRT may result in tachycardia-induced cardiomyopathy that usually resolves after successful treatment. Another unusual accessory pathway is the atriofascicular fiber (also called a Mahaim fiber) that connects the right atrium to a fascicle of the distal right bundle branch and has decremental anterograde conduction while not allowing conduction in the retrograde direction; this pathway can allow reentrant tachycardia with a circuit that involves anterograde conduction over the accessory pathway with characteristic left bundle-branch block morphology and retrograde conduction through the AV node/His Purkinje system. Other rare accessory pathway connections that may participate in reentrant tachycardia are nodofascicular pathways (connecting the AV node to a fascicle) and nodoventricular pathways (connecting the AV node to the ventricular myocardium). Fasciculoventricular pathways, connecting a fascicle to the proximal right or left bundle branch, have also been described, although they have never been reported to participate in tachycardia. An EP study is necessary to establish the diagnosis of these rare accessory pathways.
The diagnosis of WPW syndrome is reserved for patients who demonstrate ventricular pre-excitation on their resting ECG that participates in arrhythmias. Rapid anterograde accessory pathway conduction during AF can result in SCD in patients with a manifest accessory pathway, with a 10-year risk ranging from 0.15% to 0.24%.253,254 Unfortunately, SCD may be the first presentation of patients with undiagnosed WPW. Increased risk of SCD is associated with a history of symptomatic tachycardia, multiple accessory pathways, and a shortest pre-excited R-R interval of <250 ms during AF. The risk of SCD associated with WPW appears highest in the first 2 decades of life.254–258 Antiarrhythmic drug treatment of patients with orthodromic AVRT can be directed at either the accessory pathway or the AV node, as both are key portions of the reentrant circuit. AV nodal–blocking agents may be contraindicated in patients at risk of rapid conduction down the accessory pathway during AF. Catheter ablation strategies target the accessory pathway, with high success rates.
6.1. Management of Patients With Symptomatic Manifest or Concealed Accessory Pathways
See Figure 14 for the algorithm for acute treatment of orthodromic AVRT and Figure 15 for the algorithm for ongoing management of orthodromic AVRT.

- Download figure
- Download PowerPoint
Figure 14. Acute treatment of orthodromic AVRT. Colors correspond to Class of Recommendation in Table 1; drugs listed alphabetically. *For rhythms that break or recur spontaneously, synchronized cardioversion is not appropriate. AVRT indicates atrioventricular reentrant tachycardia; ECG, electrocardiogram; and IV, intravenous.

- Download figure
- Download PowerPoint
Figure 15. Ongoing management of orthodromic AVRT. Colors correspond to Class of Recommendation in Table 1; drugs listed alphabetically. AVRT indicates atrioventricular reentrant tachycardia; ECG, electrocardiogram; pt, patient; and SHD, structural heart disease (including ischemic heart disease).
6.1.1. Acute Treatment: Recommendations
| COR | LOE | Recommendations |
|---|---|---|
| I | B-R | 1. Vagal maneuvers are recommended for acute treatment in patients with orthodromic AVRT.42,75,235,259 |
| See Online Data Supplements 11 and 12. | For acute conversion of orthodromic AVRT, vagal maneuvers, including Valsalva and carotid sinus massage, can be performed quickly and should be the first-line intervention to terminate SVT. These maneuvers should be performed with the patient in the supine position. There is no "gold standard" for proper Valsalva maneuver technique, but in general, the patient raises intrathoracic pressure by bearing down against a closed glottis for 10 to 30 seconds, equivalent to at least 30 to 40 mm Hg.82,84 Carotid massage is performed after absence of bruit has been confirmed by auscultation, by applying steady pressure over the right or left carotid sinus for 5 to 10 seconds.83,84 Another vagal maneuver based on the classic diving reflex consists of applying an ice-cold, wet towel to the face85; in a laboratory setting, facial immersion in water at 10°C (50°F) has proved effective in terminating tachycardia, as well.86 One study involving 148 patients with SVT demonstrated that Valsalva was more successful than carotid sinus massage, and switching from 1 technique to the other resulted in an overall success rate of 27.7%.82 The practice of applying pressure to the eyeball is potentially dangerous and has been abandoned. | |
| I | B-R | 2. Adenosine is beneficial for acute treatment in patients with orthodromic AVRT.42,260,261 |
| See Online Data Supplements 11 and 12. | Adenosine is effective for conversion of orthodromic AVRT in 90% to 95% of patients, with minor and brief (<1 min) side effects occurring in approximately 30% of patients.42,260,261 Patients often have atrial or ventricular premature complexes immediately after conversion that, on occasion, may induce further episodes of AVRT. In this situation, an antiarrhythmic drug may be required to prevent acute reinitiation of tachycardia. Because adenosine may precipitate AF that may then conduct rapidly to the ventricle and even cause ventricular fibrillation, electrical cardioversion should be available. | |
| I | B-NR | 3. Synchronized cardioversion should be performed for acute treatment in hemodynamically unstable patients with AVRT if vagal maneuvers or adenosine are ineffective or not feasible.75,262,263 |
| See Online Data Supplement 10. | Synchronized cardioversion is highly effective in terminating AVRT.75 Cardioversion avoids complications associated with antiarrhythmic drug therapy and should be considered early in the management of hemodynamically unstable patients. Patients often have atrial or ventricular premature complexes immediately after cardioversion that, on occasion, may induce further episodes of AVRT. In this situation, an antiarrhythmic drug may be required to prevent acute reinitiation of tachycardia. | |
| I | B-NR | 4. Synchronized cardioversion is recommended for acute treatment in hemodynamically stable patients with AVRT when pharmacological therapy is ineffective or contraindicated.87,95 |
| See Online Data Supplements 3 and 10. | Synchronized cardioversion is highly effective in terminating SVT (including AVRT and AVNRT), and when the patient is stable, this is performed after adequate sedation or anesthesia.94 Most stable patients with SVT respond to pharmacological therapy, with success rates of 80% to 98% for agents such as verapamil, diltiazem, or adenosine. In some resistant cases, a second drug bolus or higher dose of initial drug agent might prove effective.87,96 Nevertheless, in rare instances, drugs may fail to successfully restore sinus rhythm. | |
| I | B-NR | 5. Synchronized cardioversion should be performed for acute treatment in hemodynamically unstable patients with pre-excited AF.75,94 |
| See Online Data Supplement 10. | Synchronized cardioversion is highly effective in terminating pre-excited AF.75 When AF occurs in patients with ventricular pre-excitation, if the accessory pathway has a short refractory period, this may allow for rapid pre-excited AV conduction; the resulting fast, often irregular, broad-complex tachycardia is often unstable and may lead to ventricular fibrillation. It is therefore important to achieve early restoration of sinus rhythm in these patients. Patients often have atrial or ventricular premature complexes immediately after cardioversion that, on occasion, may induce AVRT or recurrent pre-excited AF. | |
| I | C-LD | 6. Ibutilide264 or intravenous procainamide265 is beneficial for acute treatment in patients with pre-excited AF who are hemodynamically stable. |
| See Online Data Supplements 11 and 12. | Small observational studies support the use of ibutilide or intravenous procainamide for the treatment of pre-excited AF in patients who are not hemodynamically compromised.264,265 Both medications can decrease ventricular rate by slowing conduction over the accessory pathway and have the additional benefit of possibly terminating AF.264,265 | |
| IIa | B-R | 1. Intravenous diltiazem, verapamil42,260,266,267 (Level of Evidence: B-R ), or beta blockers268 (Level of Evidence: C-LD) can be effective for acute treatment in patients with orthodromic AVRT who do not have pre-excitation on their resting ECG during sinus rhythm. |
| C-LD | ||
| See Online Data Supplements 11 and 12. | Intravenous diltiazem or verapamil effectively terminate approximately 90% to 95% of AVRT episodes in patients without pre-excitation on their resting sinus-rhythm ECG, with drug-induced hypotension occurring in approximately 3% of patients.42,260,266,267 Intravenous beta blockers have not been studied in clinical trials; however, clinical experience suggests they are useful for terminating AVRT, with a low risk of associated complications.268 | |
| IIb | B-R | 1. Intravenous beta blockers, diltiazem, or verapamil might be considered for acute treatment in patients with orthodromic AVRT who have pre-excitation on their resting ECG and have not responded to other therapies.42,266,267,269 |
| See Online Data Supplements 11 and 12. | Intravenous beta blockers, diltiazem, and verapamil have a risk of enhancing conduction over the accessory pathway if the AVRT converts to AF during administration of the medication. Should the patient have a rapidly conducting manifest accessory pathway, further enhancing accessory-pathway conduction during AF by shortening the refractory period (digoxin) or decreasing BP and increasing catecholamines (diltiazem, beta blockers, verapamil) may place the patient at risk of AF degenerating into a malignant ventricular arrhythmia. The ability to promptly perform electrical cardioversion must be available should AF with rapid ventricular conduction occur. Before intravenous beta blockers, diltiazem, and verapamil were available, intravenous digoxin was commonly used for acute treatment of patients with orthodromic AVRT who had pre-excitation on their resting ECG270; this agent is rarely used now because other agents are available and digoxin may put patients at risk of ventricular fibrillation.271 | |
| III: Harm | C-LD | 1. Intravenous digoxin, intravenous amiodarone, intravenous or oral beta blockers, diltiazem, and verapamil are potentially harmful for acute treatment in patients with pre-excited AF.269,271–275 |
| See Online Data Supplements 11 and 12. | Patients with pre-excited AF should not receive intravenous digoxin, intravenous amiodarone, or intravenous/oral beta blockers, diltiazem, or verapamil because these medications may enhance conduction over the accessory pathway, increase the ventricular rate, and increase the risk of provoking a life-threatening ventricular arrhythmia.269,271–275 Digoxin increases the ventricular rate by shortening refractoriness of the accessory pathway, whereas amiodarone, beta blockers, diltiazem, and verapamil may increase the ventricular rate as a result of drug-induced hypotension with increased catecholamines. In addition, these medications may enhance conduction over the accessory pathway by slowing or blocking conduction through the AV node, preventing competitive concealed retrograde conduction into the accessory pathway. | |
6.1.2. Ongoing Management: Recommendations
| COR | LOE | Recommendations |
|---|---|---|
| I | B-NR | 1. Catheter ablation of the accessory pathway is recommended in patients with AVRT and/or pre-excited AF.103,254,276–282 |
| See Online Data Supplements 11 and 12. | Several large series support the use of catheter ablation of the accessory pathway as first-line therapy in patients who have had AF and/or AVRT. These series report a success rate of approximately 93% to 95% and a 3% risk of major complications when patients are followed up for 6 months to 8 years102,103,254,276–282 (Table 8). AF in younger patients is usually associated with the accessory pathway and is unlikely to occur after ablation; in contrast, older patients may have recurrence of AF from causes unrelated to the accessory pathway.283,284 Catheter ablation is also effective for treating PJRT (Table 3) by ablating the concealed accessory pathway with a success rate of approximately 90%.283,284 Catheter ablation of an atriofascicular (Mahaim) pathway is successful in preventing reentrant tachycardia in approximately 70% to 100% of patients.285,286 | |
| I | C-LD | 2. Oral beta blockers, diltiazem, or verapamil are indicated for ongoing management of AVRT in patients without pre-excitation on their resting ECG.46,287 |
| See Online Data Supplements 11 and 12. | Observational studies and clinical experience confirm that beta blockers, diltiazem, and verapamil are effective for preventing recurrent tachycardia in approximately 50% of patients without pre-excitation on their resting ECG (concealed accessory pathway) and are associated with a favorable side effect profile.46,287 | |
| IIa | B-R | 1. Oral flecainide or propafenone is reasonable for ongoing management in patients without structural heart disease or ischemic heart disease who have AVRT and/or pre-excited AF and are not candidates for, or prefer not to undergo, catheter ablation.45,108,109,112,288 |
| See Online Data Supplements 11 and 12. | Flecainide and propafenone are beneficial for the treatment of AVRT by directly slowing or blocking conduction over the pathway. These drugs are effective in approximately 85% to 90% of patients, with 30% reporting an absence of tachycardia.45,108,109,112,288 Both drugs have a risk of proarrhythmia resulting in VT that is increased in patients with structural heart disease or ischemic heart disease; in such patients, these drugs are generally avoided. Side effects occur in up to 60% of patients, and approximately 20% discontinue the medications because of adverse effects.45,108,109,112,288 | |
| IIb | B-R | 1. Oral dofetilide or sotalol may be reasonable for ongoing management in patients with AVRT and/or pre-excited AF who are not candidates for, or prefer not to undergo, catheter ablation.99,106 |
| See Online Data Supplement 9. | Unlike flecainide and propafenone, sotalol and dofetilide can be used in patients with structural heart disease or coronary artery disease.105 Given the potential for significant QT prolongation and torsades de pointes, inpatient monitoring with serial ECGs is generally performed when these agents are initiated. | |
| IIb | C-LD | 2. Oral amiodarone may be considered for ongoing management in patients with AVRT and/or pre-excited AF who are not candidates for, or prefer not to undergo, catheter ablation and in whom beta blockers, diltiazem, flecainide, propafenone, and verapamil are ineffective or contraindicated.289,290 |
| See Online Data Supplements 11 and 12. | Small observational studies support the use of amiodarone for preventing recurrent AVRT, but long-term efficacy has not been reported.289,290 Because of the important toxicity associated with long-term use of amiodarone, the drug is usually reserved for patients who are not candidates for catheter ablation and who have failed to respond to or have contraindications to other antiarrhythmic drugs. | |
| IIb | C-LD | 3. Oral beta blockers, diltiazem, or verapamil may be reasonable for ongoing management of orthodromic AVRT in patients with pre-excitation on their resting ECG who are not candidates for, or prefer not to undergo, catheter ablation.46,287 |
| See Online Data Supplements 11 and 12. | One RCT supports the use of verapamil for prevention of orthodromic AVRT in patients with pre-excitation on their resting ECG (manifest accessory pathway).46 There are no RCTs supporting the use of oral beta blockers or diltiazem for prevention of recurrent AVRT, although clinical experience suggests the drugs are effective, with a favorable side effect profile.287 Patients with pre-excitation may develop AF during an episode of AVRT and be exposed to increased risk of rapid conduction over the accessory pathway while receiving beta blockers, diltiazem or verapamil, so these agents must be used with caution.269 The decision to treat with these agents should follow a discussion of risks with the patient. Although evidence of poor anterograde conduction via the accessory pathway may be reassuring, rapid conduction in AF has been described even in the setting of intermittent anterograde conduction.291 | |
| IIb | C-LD | 4. Oral digoxin may be reasonable for ongoing management of orthodromic AVRT in patients without pre-excitation on their resting ECG who are not candidates for, or prefer not to undergo, catheter ablation.292 |
| See Online Data Supplement 12. | One small study reported the usefulness of oral digoxin in prevention of recurrent orthodromic AVRT in patients without pre-excitation on their resting ECG (concealed accessory pathway).293 Digoxin has been used clinically for many years, but the low efficacy indicates its use would be best limited to patients who are not candidates for catheter ablation or prefer pharmacological therapy and have failed to respond to other antiarrhythmic drugs. | |
| III: Harm | C-LD | 1. Oral digoxin is potentially harmful for ongoing management in patients with AVRT or AF and pre-excitation on their resting ECG.271 |
| See Online Data Supplement 12. | Digoxin shortens the refractory period of the accessory pathway, such that AF may induce ventricular fibrillation.271 Even if AF has never been documented, AVRT may degenerate into AF. Thus, oral digoxin should not be used to treat patients with a manifest accessory pathway. | |
6.2. Management of Asymptomatic Pre-Excitation
6.2.1. PICOTS Critical Questions
See the ERC systematic review report, "Risk Stratification for Arrhythmic Events in Patients With Asymptomatic Pre-Excitation" for the complete evidence review on the management of asymptomatic pre-excitation,9 and see Online Data Supplements 13, 14, and 15 for additional data on asymptomatic pre-excitation, which were reproduced directly from the ERC's systematic review. These recommendations have been designated with the notation SR to emphasize the rigor of support from the ERC's systematic review. PICOTS Question 1 did not provide adequate data for a recommendation; the other 3 PICOTS questions are addressed in the recommendations in Section 6.2.2.
As noted in Section 1.1, the recommendations in Section 6.3 are based on a separately commissioned systematic review of the available evidence, the results of which were used to frame our decision making. Full details are provided in the ERC's systematic review report.9 The following 4 questions were considered by the ERC:
-
What is the comparative predictive accuracy of invasive EP study (without catheter ablation of the accessory pathway) versus noninvasive testing for predicting arrhythmic events (including SCD) in patients with asymptomatic pre-excitation?
-
What is the usefulness of invasive EP study (without catheter ablation of the accessory pathway) versus no testing for predicting arrhythmic events (including SCD) in patients with asymptomatic pre-excitation?
-
What is the usefulness of invasive EP study (without catheter ablation of the accessory pathway) or noninvasive EP study for predicting arrhythmic events (including SCD) in patients with asymptomatic pre-excitation?
-
What are the efficacy and effectiveness of invasive EP study with catheter ablation of the accessory pathway as appropriate versus noninvasive tests with treatment (including observation) or no testing/ablation as appropriate for preventing arrhythmic events (including SCD) and improving outcomes in patients with asymptomatic pre-excitation?
6.2.2. Asymptomatic Patients With Pre-Excitation: Recommendations
| COR | LOE | Recommendations |
|---|---|---|
| I | B-NR SR | 1. In asymptomatic patients with pre-excitation, the findings of abrupt loss of conduction over a manifest pathway during exercise testing in sinus rhythm294–297 (Level of Evidence: B-NR) SR or intermittent loss of pre-excitation during ECG or ambulatory monitoring297 (Level of Evidence: C-LD) SR are useful to identify patients at low risk of rapid conduction over the pathway. |
| C-LD SR | ||
| See Online Data Supplements 11 and 12. | Noninvasive testing has been shown to identify patients at low risk of developing rapid conduction over the accessory pathway and life-threatening ventricular arrhythmias in response to AF. The noninvasive findings that identify a pathway not capable of maintaining rapid conduction during AF include intermittent loss of conduction over the accessory pathway on the resting ECG or during ambulatory monitoring, or abrupt loss of pre-excitation during exercise testing (Figure 16).294–297 The ECG should be evaluated closely to make certain the delta wave is truly absent, as accessory pathways, especially left lateral pathways, may demonstrate varying degrees of pre-excitation because of fusion between conduction over the accessory pathway and through the AV node. This may give the appearance of loss of pre-excitation if the subtle delta wave is not identified. Noninvasive tests have an approximately 90% positive predictive value and 30% negative predictive value for identifying pathways with life-threatening properties.294,295,297 | |
| IIa | B-NR SR | 1. An EP study is reasonable in asymptomatic patients with pre-excitation to risk-stratify for arrhythmic events.254,256,298–301 |
| See Online Data Supplements 11–15. | In the absence of symptoms, a clinical priority is identifying accessory pathways at increased risk of arrhythmic events, including rapid conduction during AF and development of life-threatening ventricular arrhythmias, with the most useful findings being the following: an R-R interval <250 ms between 2 pre-excited complexes during induced AF; the presence of multiple accessory pathways; the ability to induce sustained AVRT; the finding of AVRT precipitating pre-excited AF; and an accessory pathway refractory period <240 ms.254,256,298,299,301 Malignant arrhythmias correlate more with the EP properties of the accessory pathway than with the presence or absence of symptoms. This approach is supported by the low risk of complications observed in an EP study in which complication rates among 2169 patients ranged from 0.09% to 1% and included pneumothorax and access site complications.254 | |
| IIa | B-NR SR | 2. Catheter ablation of the accessory pathway is reasonable in asymptomatic patients with pre-excitation if an EP study identifies a high risk of arrhythmic events, including rapidly conducting pre-excited AF.254,302,303 |
| See Online Data Supplements 11–15. | In a large prospective cohort study of 756 asymptomatic patients with close to 8 years of follow-up, 9% of patients developed malignant AF (shortest R-R interval ≤250 ms), and 2% developed ventricular fibrillation.254 Malignant arrhythmias correlated more with high-risk EP properties of the accessory pathway than with the presence or absence of symptoms. Ablation of the accessory pathway(s) in high-risk patients was also examined in 1 RCT that enrolled 76 patients, showing that arrhythmic events (defined as symptomatic SVT, AF, and ventricular fibrillation in this study) occurred in 7% of patients who underwent ablation versus 77% who did not undergo ablation.302 Another study that examined patients on the basis of whether an ablation was performed reported that none of the asymptomatic patients who had undergone ablation of the accessory pathway developed a malignant arrhythmia during 8 years of follow-up. The risk of complications with ablation ranged from 0.1% (complete heart block) to 0.9% (ablation-induced right bundle-branch block).254 The risks and benefits of proceeding with ablation of pathways found not to have high-risk characteristics should be discussed thoroughly with patients in advance of the EP procedure. | |
| IIa | B-NR SR | 3. Catheter ablation of the accessory pathway is reasonable in asymptomatic patients if the presence of pre-excitation precludes specific employment (such as with pilots).103,254,276–282,302–304 |
| See Online Data Supplements 11–15. | Patients with asymptomatic pre-excitation whose job activities would place them or others at risk if a hemodynamically significant arrhythmia occurred (such as airline pilots) are potential candidates for catheter ablation. Catheter ablation is associated with a success rate of approximately 95% and a 3% risk of major complications when patients are followed up for 6 months to 8 years.103,254,276–282,302,303 Other documents advise EP study in asymptomatic athletes who engage in moderate- or high-level competitive sports.305 | |
| IIa | B-NR SR | 4. Observation, without further evaluation or treatment, is reasonable in asymptomatic patients with pre-excitation.301,306–309 |
| See Online Data Supplements 11–15. | Most observational cohort studies suggest that the great majority of adult patients with asymptomatic pre-excitation who do not undergo an ablation of the accessory pathway have a benign course with few clinically significant arrhythmic events occurring over time. This supports the recommendation that observation without medical therapy or ablation is a reasonable alternative because the risk of SCD is small and is seen mainly in children.254,301,306–309 The choice to observe asymptomatic patients should be preceded by the patient being informed of the small risk of life-threatening arrhythmias developing in the absence of treatment, along with the success rate and complications associated with catheter ablation of the accessory pathway. | |
6.3. Risk Stratification of Symptomatic Patients With Manifest Accessory Pathways: Recommendations
| COR | LOE | Recommendations |
|---|---|---|
| I | B-NR | 1. In symptomatic patients with pre-excitation, the findings of abrupt loss of conduction over the pathway during exercise testing in sinus rhythm294–297 (Level of Evidence: B-NR) or intermittent loss of pre-excitation during ECG or ambulatory monitoring297 (Level of Evidence: C-LD) are useful for identifying patients at low risk of developing rapid conduction over the pathway. |
| C-LD | ||
| See Online Data Supplements 11–15. | An important consideration in the evaluation of patients with pre-excitation is determining risk of developing rapid conduction over the accessory pathway and life-threatening ventricular arrhythmias in response to AF. The noninvasive findings that identify a pathway incapable of maintaining rapid conduction during AF include intermittent loss of conduction over the accessory pathway on the resting ECG or during ambulatory monitoring or abrupt loss of pre-excitation during exercise testing.294–297 The ECG should be evaluated closely to make certain the delta wave is truly absent, as accessory pathways (especially left lateral pathways) may demonstrate varying degrees of pre-excitation because of fusion between conduction over the accessory pathway and through the AV node, which may give the appearance of loss of pre-excitation if the subtle delta wave is not identified. Noninvasive tests have an approximately 90% positive predictive value and 30% negative predictive value for identifying pathways with life-threatening properties.294,295,297 If noninvasive evaluation suggests that the accessory pathway conducts poorly in the anterograde direction, although risk of life-threatening events is likely lower, the EP study still may be useful because of patient symptoms. | |
| I | B-NR | 2. An EP study is useful in symptomatic patients with pre-excitation to risk-stratify for life-threatening arrhythmic events.254,256,298–300 |
| See Online Data Supplements 11–15. | Most symptomatic patients with accessory pathways undergo catheter ablation, but in some instances, EP studies are performed to identify whether the patient is at increased risk of rapid conduction down the accessory pathway during AF and development of life-threatening ventricular arrhythmias. The most useful findings for risk stratification are an R-R interval <250 ms between 2 pre-excited complexes during induced AF; the presence of multiple accessory pathways; the finding of AVRT precipitating pre-excited AF; and an accessory pathway refractory period <240 ms.254,256,298–300 | |
7. Atrial Flutter
See Figure 17 for a schematic depicting classification of atrial flutter/ATs; Figure 18 for the algorithm for acute treatment of atrial flutter; and Figure 19 for the algorithm for ongoing management of atrial flutter.

- Download figure
- Download PowerPoint
Figure 16. Abrupt loss of pre-excitation during exercise testing. During exercise treadmill testing, this patient abruptly lost pre-excitation at a heart rate of 117 bpm. Beginning abruptly with the beat under the arrow, the PR interval normalizes, and the QRS changes from pre-excited to narrow.

- Download figure
- Download PowerPoint
Figure 17. Classification of atrial flutter/atrial tachycardias. Diagram summarizing types of ATs often encountered in patients with a history of atrial fibrillation, including those seen after catheter or surgical ablation procedures. P-wave morphologies are shown for common types of atrial flutter; however, the P-wave morphology is not always a reliable guide to the re-entry circuit location or to the distinction between common atrial flutter and other macroreentrant ATs. *Exceptions to P-wave morphology and rate are common in scarred atria. bpm indicates beats per minute, and ECG, electrocardiogram.
Reproduced with permission from January et al.10

- Download figure
- Download PowerPoint
Figure 18. Acute treatment of atrial flutter. Colors correspond to Class of Recommendation in Table 1; drugs listed alphabetically. *Anticoagulation as per guideline is mandatory. †For rhythms that break or recur spontaneously, synchronized cardioversion or rapid atrial pacing is not appropriate. IV indicates intravenous.

- Download figure
- Download PowerPoint
Figure 19. Ongoing management of atrial flutter. Colors correspond to Class of Recommendation in Table 1; drugs listed alphabetically. *After assuring adequate anticoagulation or excluding left atrial thrombus by transesophageal echocardiography before conversion. †Should be combined with AV nodal–blocking agents to reduce risk of 1:1 conduction during atrial flutter. AV indicates atrioventricular; SHD, structural heart disease (including ischemic heart disease).
7.1. Cavotricuspid Isthmus–Dependent Atrial Flutter
Atrial flutter is a macroreentrant atrial arrhythmia characterized by regular atrial rate and constant P-wave morphology. When the atrial flutter circuit involves the cavotricuspid isthmus (CTI), it is labeled CTI-dependent atrial flutter. When CTI-dependent flutter involves a circuit that rotates around the tricuspid valve in a counterclockwise direction (up the septum and down the free wall), it is called "typical"; less commonly, the CTI-dependent flutter circuit rotates in a clockwise direction (sometimes called "reverse typical").203 Counterclockwise CTI-dependent atrial flutter is characterized electrocardiographically by dominant negative flutter waves in the inferior leads (so-called "sawtooth waves") and a positive P wave in lead V1 at atrial rates of 250 bpm to 350 bpm (Figure 17). Clockwise isthmus-dependent flutter shows the opposite pattern (ie, positive flutter waves in the inferior leads and wide, negative flutter waves in lead V1) (Figure 17). Although the atrial rates for flutter typically range from 250 bpm to 330 bpm, the rates may be slower in patients with severe atrial disease or in patients taking antiarrhythmic agents or after unsuccessful catheter ablation.310
Atrial flutter can occur in clinical settings similar to those associated with AF, and atrial flutter can be triggered by AT or AF.121,311 It is common for AF and atrial flutter to coexist in the same patient. After CTI ablation, 22% to 50% of patients have been reported to develop AF after a mean follow-up of 14 to 30 months, although 1 study reported a much higher rate of AF development, with 82% of patients treated by catheter ablation for atrial flutter manifesting AF within 5 years.312 Risk factors for the manifesting AF after atrial flutter ablation include prior AF, depressed left ventricular function, structural heart disease or ischemic heart disease, inducible AF, and increased LA size.121,312–316
Atrial flutter may result from antiarrhythmic therapy of AF, particularly when flecainide, propafenone, or amiodarone is used for treatment of AF.317,318 In those patients with atrial flutter resulting from antiarrhythmic therapy of AF, ablation of the CTI-dependent flutter may prevent recurrent flutter while antiarrhythmic therapy for AF is continued.318
Patients with atrial flutter are thought to have the same risk of thromboembolism as patients with AF; therefore, recommendations for anticoagulation mirror those for patients with AF.10,121,314 Similarly, the recommendations for anticoagulation with regard to either pharmacological or electrical cardioversion of patients with atrial flutter are the same as those for patients with AF, as discussed in the 2014 AF guideline (Section 6.1).10
7.2. Non–Isthmus-Dependent Atrial Flutters
Non–isthmus-dependent atrial flutter or atypical flutter describes macroreentrant ATs that are not dependent on conduction through the CTI. A variety of circuits have been described, including a path around the mitral annulus (perimitral flutter), re-entry involving the left atrial roof, and re-entry around regions of scarring in the right or left atrium. Non–isthmus-dependent atrial flutters often occur in patients with atrial scarring from prior heart surgery or ablation but also may occur in any form of heart disease or may be idiopathic.134,140,319 Non–isthmus-dependent atrial flutters can coexist with CTI-dependent flutter or involve the presence of multiple atrial re-entry circuits.133,320 The reentrant circuits are classified as either macroreentrant AT (large; often several centimeters or longer in diameter) or microreentrant AT (≤2 cm in diameter), which may be indistinguishable from focal AT.321
In the presence of substantial atrial disease, prior surgery, or prior radiofrequency catheter ablation, the ECG flutter-wave morphology is not a reliable predictor of whether the flutter circuit involves the CTI. Although an ECG with a typical flutter appearance has good predictive value for CTI-dependent flutter in a patient who has not undergone prior catheter ablation of AF, the ECG appearance is less useful in predicting the flutter circuit in a patient who has previously undergone AF ablation.322–325 The presence of a positive or biphasic (but dominantly positive) deflection in V1, accompanied by deflections in other leads inconsistent with typical counterclockwise atrial flutter, suggests the presence of an atypical flutter (Figure 17). Definitive diagnosis requires EP study and intracardiac mapping.326
Catheter ablation of non–CTI-dependent flutter requires more extensive mapping than does ablation of CTI-dependent flutter, and success rates are lower (Table 8). The location of the circuit determines ablation approach and risks.
| Arrhythmia | Acute Success | Recurrence Rate | Major Complications | References |
|---|---|---|---|---|
| Common SVTs | ||||
| AVNRT | 96%–97%102,103 | 5%103 | • Overall 3%102 | 102,103 |
| • PPM 0.7%102 | ||||
| • Death 0%102 | ||||
| AVRT/accessory pathway | 93%102,103 | 8%103 | • Overall 2.8%102 | 102,103 |
| • PPM 0.3%102 | ||||
| • Death 0.1%102 | ||||
| • Tamponade 0.4%102 | ||||
| CTI-dependent atrial flutter | 97%102 | 10.6% atrial flutter,121 33% atrial fibrillation121 | • Overall 0.5%102 | 102,103,121 |
| • PPM 0.2%102 | ||||
| • Pericardial effusion 0.3%102 | ||||
| Less common SVTs | ||||
| Focal AT | 80%–100% | 4%–27% | <1%–2% | 122–129 |
| JT | 82%–85% | 0–18% | 0–18% CHB (overall complications N/A) | 130–132 |
| Non–CTI-dependent atrial flutter | 73%–100% | 7%–53% | 0–7% | 122,133–140 |
The substrate for macroreentrant atrial arrhythmias after cardiac surgery is atrial scarring from atriotomy incisions and cannulation sites and from the underlying myopathic process of the valve disease itself; this is sometimes referred to as incisional atrial reentrant tachycardia. The location of the reentrant circuit depends on the type of surgical approach, and common populations include patients who have undergone mitral valve surgery or have a repaired atrial septal defect.327–330 These arrhythmias are also common after surgical or catheter ablation for AF.331,332 Both single- and dual-loop circuits, as well as focal ATs, can be present. It is useful to review the procedural notes to identify the location of atrial incisions or prior ablation that can assist with future mapping and ablation.
The development of a microreentrant or macroreentrant left AT after AF ablation occurs in approximately 5% of patients.330,333,334 This is less frequent if ablation is limited to pulmonary vein isolation. On the other hand, these arrhythmias are more common in patients with longer-duration persistent AF or more dilated left atria or when linear ablation lesions are used.333–338 Non–reentrant focal arrhythmias often originate at lesion edges or reconnected segments of prior isolated pulmonary veins.333 Reisolation of the pulmonary vein and ablation of any nonpulmonary vein foci are often effective in treating these arrhythmias. Detailed activation and entrainment mapping of the tachycardia during a second procedure result in effective ablation in approximately 90% of patients.335 Although right atrial CTI-dependent flutter may also occur, most of the tachycardias originate in the left atrium.
As with all types of atrial flutter, it may be very difficult to achieve rate control in patients with post–AF ablation non–CTI-dependent flutter (far more so than in patients with preablation AF). When the ventricular response cannot be controlled with common rate-control medications, attempts at restoration of sinus rhythm with pharmacological therapy and cardioversion are often required. Many of the atrial flutters that are observed during the first 3 months after catheter ablation or after cardiac surgery will not recur later on. For this reason, it is advised that attempts at ablation of atrial flutter after AF ablation be deferred until after the 3-month waiting period.339 Rarely, pharmacological therapy and attempts at rhythm control with antiarrhythmic drug therapy fail to adequately control atrial flutter during the 3 months after AF ablation. In this situation, early repeat ablation is warranted.
7.3. Acute Treatment: Recommendations
| COR | LOE | Recommendations |
|---|---|---|
| I | A | 1. Oral dofetilide or intravenous ibutilide is useful for acute pharmacological cardioversion in patients with atrial flutter.119,340–346 |
| See Online Data Supplements 16 and 17. | Pharmacological cardioversion of atrial flutter is generally less effective than synchronized cardioversion and carries the potential risk of proarrhythmia but can be an option when sedation is not available or not well tolerated or when indicated by patient preference. Intravenous ibutilide converts atrial flutter to sinus rhythm in approximately 60% of cases.342 The major risk is torsades de pointes, which is more likely to occur in patients with reduced left ventricular ejection fraction. Patients receiving ibutilide should undergo continuous ECG monitoring during administration and for at least 4 hours after completion of dosing. Pretreatment with magnesium can increase the efficacy and reduce the risk of torsades de pointes.119 Anticoagulation issues for chemical cardioversion are the same as those for electrical cardioversion of atrial flutter.10 | |
| I | B-R | 2. Intravenous or oral beta blockers, diltiazem, or verapamil are useful for acute rate control in patients with atrial flutter who are hemodynamically stable.347–354 |
| See Online Data Supplements 16 and 17. | It is often more difficult to achieve rate control for atrial flutter than for AF. Nonetheless, effective rate control may be achieved with beta blockers, diltiazem, or verapamil in patients with atrial flutter through a direct effect on the AV node. Hypotension is the main adverse effect. Intravenous diltiazem is the preferred intravenous calcium channel blocker for acute rate control because of its safety and efficacy.351 Diltiazem and verapamil should be avoided in patients with advanced heart failure and in patients with heart block or sinus node dysfunction in the absence of pacemaker therapy. Verapamil and diltiazem also should not be used in patients with known pre-excitation. The heart rate control achieved with beta blockers is similar to that seen with verapamil and diltiazem. The rate-slowing effect of beta blockers is largely related to reduction of sympathetic tone. Esmolol is generally the preferred intravenous beta blocker because of its rapid onset.353 Care should be used in administering beta blockers for rate control in atrial flutter patients with decompensated heart failure or reactive airway disease.355 | |
| I | B-NR | 3. Elective synchronized cardioversion is indicated in stable patients with well-tolerated atrial flutter when a rhythm-control strategy is being pursued.356–358 |
| See Online Data Supplements 16 and 17. | Heart rates can be difficult to control in atrial flutter. Cardioversion for atrial flutter can be successful at lower energy levels than for AF.356 Anticoagulation issues for cardioversion are the same as those for patients with AF and are addressed in the 2014 AF guideline.10 Restoration of sinus rhythm is favored to avoid development of tachycardia-mediated cardiomyopathy, which is associated with a rapid ventricular response in atrial flutter. | |
| I | B-NR | 4. Synchronized cardioversion is recommended for acute treatment of patients with atrial flutter who are hemodynamically unstable and do not respond to pharmacological therapies.75,208,356,359 |
| See Online Data Supplements 16 and 17. | The ventricular rate in atrial flutter can be difficult to control with pharmacological therapy. In patients with any signs or symptoms of hemodynamic compromise attributed to atrial flutter, synchronized cardioversion (with appropriate considerations with regard to anticoagulation) should be pursued without delay. | |
| I | C-LD | 5. Rapid atrial pacing is useful for acute conversion of atrial flutter in patients who have pacing wires in place as part of a permanent pacemaker or implantable cardioverter-defibrillator or for temporary atrial pacing after cardiac surgery.360–364 |
| See Online Data Supplements 16 and 17. | Atrial pacing is effective at terminating flutter in >50% of cases.364 Atrial pacing is more commonly applied in situations in which atrial wires are already in place, such as in the postoperative setting or in patients with programmable cardiac implanted electrical devices. A temporary pacing wire may also be placed and atrial pacing for termination of atrial flutter can be useful when sedation is contraindicated, or in the setting of digitalis toxicity, in which DC cardioversion is contraindicated. Pace-termination is performed by pacing the atrium at a rate starting approximately 5% to 10% above the atrial flutter rate to achieve atrial entrainment and by maintaining pacing for ≥15 seconds, with repeated attempts at incrementally faster rates (reducing the pacing cycle length by 5 to 10 ms until normal sinus rhythm or AF occurs.364 When AF is precipitated, this is often more easily rate-controlled and may subsequently revert to sinus rhythm. Recommendations for anticoagulation with regard to pace-termination of atrial flutter are the same as those for chemical or electrical conversion of AF. | |
| I | B-NR | 6. Acute antithrombotic therapy is recommended in patients with atrial flutter to align with recommended antithrombotic therapy for patients with AF.365 |
| See Online Data Supplements 16 and 17. | The risk of stroke associated with AF is well established, and several RCTs have demonstrated the efficacy of anticoagulation for stroke prevention in patients with additional risk factors.366 Anticoagulation has also been shown to prevent stroke when administered during the weeks immediately before and after cardioversion.367 Whether atrial flutter carries a risk of stroke similar to that in patients with AF has been debated in the past but is now supported by limited evidence from mechanistic, observational, and prospective studies that include patients primarily with atrial flutter.365–369 Several reports have suggested that the risk of stroke in patients with atrial flutter is mitigated by anticoagulation. Meta-analysis of 13 studies of patients undergoing cardioversion of atrial flutter reported short-term stroke risks ranging from 0% to 7%, and the thromboembolism rate in patients with sustained flutter in 4 studies averaged 3% annually.365 Other studies have reported similar risk and effectiveness of anticoagulation.369 Therefore, on the basis of the available data, recommendations for antithrombotic therapy for patients with atrial flutter are similar to those for patients with AF.10 | |
| IIa | B-R | 1. Intravenous amiodarone can be useful for acute control of the ventricular rate (in the absence of pre-excitation) in patients with atrial flutter and systolic heart failure, when beta blockers are contraindicated or ineffective.350,370,371 |
| See Online Data Supplements 16 and 17. | Amiodarone may be useful for rate control in non–pre-excited atrial flutter because of its slowing of conduction through the AV node and prolongation of AV nodal refractoriness. Because it has less negative inotropic effect than beta blockers, diltiazem, and verapamil and may produce less hypotension, it may be preferred in critically ill patients or in those with tenuous hemodynamic stability. Because of potential toxicity, amiodarone should not be used for long-term rate control in most patients. Although unlikely, amiodarone may result in conversion of atrial flutter to sinus rhythm, so potential risks and benefits should be considered for patients with atrial flutter lasting ≥48 hours who are not adequately anticoagulated.10 However, amiodarone is typically used for rate control only when other options are greatly limited. For patients with atrial flutter, antithrombotic therapy is recommended according to the same risk profile used for AF. | |
7.4. Ongoing Management: Recommendations
| COR | LOE | Recommendations |
|---|---|---|
| I | B-R | 1. Catheter ablation of the CTI is useful in patients with atrial flutter that is either symptomatic or refractory to pharmacological rate control.243,372–375 |
| See Online Data Supplements 16 and 17. | Rate control can be difficult to achieve in atrial flutter, and a rhythm control strategy is often chosen. Catheter ablation of CTI-dependent atrial flutter is often preferred to long-term pharmacological therapy; in this rhythm, the CTI represents the optimal target for ablation because a line of ablation between the tricuspid valve annulus and inferior vena cava can effectively interrupt the circuit. A variety of techniques are applicable, including various catheter types, energy delivery systems, and mapping and visualization tools; generally, success depends on creation of a complete line of block and permanent interruption of conduction across the CTI (Table 8). Successful ablation is often heralded by intraprocedural interruption of the arrhythmia and subsequent EP demonstration of bidirectional block across the ablated tissue. | |
| I | C-LD | 2. Beta blockers, diltiazem, or verapamil are useful to control the ventricular rate in patients with hemodynamically tolerated atrial flutter.347–349 |
| See Online Data Supplements 16 and 17. | In some circumstances of persistent atrial flutter or in patients who have infrequent reasonably well-tolerated episodes of atrial flutter, a rate-control strategy may be chosen. In atrial flutter, the relatively slower atrial rate compared with AF often paradoxically results in more rapid AV nodal conduction because there is less concealed AV nodal conduction. Therefore, achieving adequate rate control can be difficult. Higher doses of beta blockers, diltiazem, or verapamil, and often a combination of agents, may be needed to achieve adequate rate control. Beta blockers are generally preferred in patients with heart failure. Avoidance of beta blockers, diltiazem, and verapamil in patients with pre-excited atrial flutter is recommended, given the potential for accelerated ventricular rates degenerating to ventricular fibrillation, as has been reported to occur rarely in similar patients with AF.274 | |
| I | C-LD | 3. Catheter ablation is useful in patients with recurrent symptomatic non–CTI-dependent flutter after failure of at least 1 antiarrhythmic agent.134,327 |
| See Online Data Supplements 16 and 17. | No prospective RCTs have compared the efficacy or safety of antiarrhythmic drugs with that of catheter ablation for non–CTI-dependent atrial flutter. In general, catheter ablation of non–CTI-dependent atrial flutter is substantially more difficult than ablation of CTI-dependent flutter because the anatomic circuits are complex, are often not anatomically defined, and can be difficult to locate. Knowledge of the prior surgical or ablation approach and detailed activation and entrainment mapping of the tachycardia are useful during attempts at ablation (Table 8). The location of the circuit determines ablation approach and risks. Observational data support the relative effectiveness and safety of catheter ablation in experienced centers.134,327 Many of the atrial flutters that are observed during the first 3 months after catheter ablation or cardiac surgery will not persist beyond the periprocedural period, so attempts at ablation can be deferred unless pharmacological therapy and/or cardioversion are unsuccessful.327 | |
| I | B-NR | 4. Ongoing management with antithrombotic therapy is recommended in patients with atrial flutter to align with recommended antithrombotic therapy for patients with AF.365 |
| See Online Data Supplements 16 and 17. | The risk of stroke associated with AF is well established, and several RCTs have demonstrated the efficacy of anticoagulation for stroke prevention in patients with additional risk factors.366 Anticoagulation has also been shown to prevent stroke when administered during the weeks immediately before and after cardioversion.367 Whether atrial flutter carries a risk of stroke similar to that in patients with AF has been debated in the past but is now supported by limited evidence from mechanistic, observational, and prospective studies that include patients primarily with atrial flutter.365–369 Several reports have suggested that the risk of stroke in patients with atrial flutter is mitigated by anticoagulation. Meta-analysis of 13 studies of patients undergoing cardioversion of atrial flutter reported short-term stroke risks ranging from 0% to 7%, and the thromboembolism rate in patients with sustained flutter in 4 studies averaged 3% annually.365 Other studies have reported similar risk and effectiveness of anticoagulation.369 Therefore, on the basis of available data, recommendations for antithrombotic therapy for patients with atrial flutter are similar to those for patients with AF.10 | |
| IIa | B-R | 1. The following drugs can be useful to maintain sinus rhythm in patients with symptomatic, recurrent atrial flutter, with the drug choice depending on underlying heart disease and comorbidities:a. Amiodarone376b. Dofetilide346,377c. Sotalol378 |
| See Online Data Supplements 16 and 17. | In patients in whom ablation is not being considered because of contraindications (such as underlying medical illness) or because of patient preference, a variety of antiarrhythmic drugs are available. These drugs act by suppressing triggers and altering atrial tissue refractoriness. Individual properties of each drug need to be considered for proper use. Much of the data pertaining to use of amiodarone in management of atrial arrhythmias has been derived from use in patients with AF.10 Few data are available for patients with atrial flutter. Amiodarone has significant toxicities, so it is used only when other treatments are contraindicated or ineffective. Nevertheless, administration is reasonable, particularly in patients with heart failure or significant underlying heart disease.376Dofetilide may be more effective than many other drugs but must be started in an inpatient setting.346,377 The dose is adjusted on the basis of renal function, with close monitoring of the Q-T interval and subsequent monitoring for altered renal function.Sotalol, a class III antiarrhythmic, is generally well tolerated but is associated with typical beta blocker side effects, such as fatigue and bradycardia.378 The major potential cardiac toxicity for both drugs is torsades de pointes. | |
| IIa | B-NR | 2. Catheter ablation is reasonable in patients with CTI-dependent atrial flutter that occurs as the result of flecainide, propafenone, or amiodarone used for treatment of AF.317,379–381 |
| See Online Data Supplements 16 and 17. | Some patients with AF treated with propafenone, flecainide, or amiodarone will develop atrial flutter. In this circumstance, if flutter becomes the dominant arrhythmia, ablation of the CTI and continued use of the antiarrhythmic drug can decrease the incidence of atrial flutter and facilitate the pharmacological management of AF.379,380 | |
| IIa | C-LD | 3. Catheter ablation of the CTI is reasonable in patients undergoing catheter ablation of AF who also have a history of documented clinical or induced CTI-dependent atrial flutter.381,382 |
| See Online Data Supplements 16 and 17. | The indications for catheter ablation of AF are discussed in the 2014 AF guideline.10 When AF and atrial flutter coexist, 1 randomized study demonstrated that at 1-year follow-up, greater success in terms of arrhythmia suppression and quality-of-life score resulted from AF ablation (with or without atrial flutter ablation) than from atrial flutter ablation alone.381 It may be that AF ablation alone is sufficient to control both arrhythmias, although CTI ablation reduced the early postablation arrhythmia recurrence rate.382 | |
| IIa | C-LD | 4. Catheter ablation is reasonable in patients with recurrent symptomatic non–CTI-dependent flutter as primary therapy, before therapeutic trials of antiarrhythmic drugs, after carefully weighing potential risks and benefits of treatment options.135 |
| See Online Data Supplements 16 and 17. | No prospective RCTs have compared the efficacy or safety of antiarrhythmic drugs with that of catheter ablation for non–CTI-dependent atrial flutter. Observational data, however, support the relative effectiveness and safety of catheter ablation in experienced centers.135,327 Many of the atrial flutters that are observed during the first 3 months after ablation or cardiac surgery will not persist beyond the periprocedural period, so attempts at ablation can be deferred unless attempts at pharmacological therapy or cardioversion are unsuccessful.135 | |
| IIb | B-R | 1. Flecainide or propafenone may be considered to maintain sinus rhythm in patients without structural heart disease or ischemic heart disease who have symptomatic recurrent atrial flutter.383–385 |
| See Online Data Supplements 16 and 17. | Data to support the recommendation for flecainide and propafenone for maintenance of sinus rhythm in patients with atrial flutter is derived from trials that pooled patients with AF and atrial flutter, with the vast majority of the patients having AF. It is therefore possible that these agents are not as effective for treating isolated atrial flutter as they are for AF.386 Flecainide and propafenone may result in slowing of the atrial flutter cycle length, which may lead to a rapid 1:1 ventricular response.387 Because of this, caution is advised with flecainide and propafenone in patients with atrial flutter at risk of 1:1 conduction. The risk may be reduced by coadministration of medications that slow AV nodal conduction, such as beta blockers, verapamil, or diltiazem. | |
| IIb | C-LD | 2. Catheter ablation may be reasonable for asymptomatic patients with recurrent atrial flutter.102,121,372 |
| See Online Data Supplements 16 and 17. | Catheter ablation of atrial flutter is highly effective, with single-procedure success rates >90%102 and an excellent safety profile.102,121 In patients with recurrent atrial flutter, long-term maintenance of sinus rhythm is more likely with ablation than with pharmacological therapy.372 Also, ablation may avoid potential development of tachycardia-mediated cardiomyopathy. | |
8. Junctional Tachycardia
See Figure 20 for a representative ECG for junctional tachycardia and Figure 21 for the algorithm for ongoing management of junctional tachycardia.

- Download figure
- Download PowerPoint
Figure 20. Surface electrocardiographic recording from leads VI, II, and V5 in a patient with junctional tachycardia. The upper panel shows sinus rhythm. The lower panel shows tachycardia onset with the characteristic finding of dissociation of the QRS and P waves (narrow arrows on P waves). The large arrow signifies continuous recording. Reproduced with permission from Blomström-Lundqvist et al.11

- Download figure
- Download PowerPoint
Figure 21. Ongoing management of junctional tachycardia. Colors correspond to Class of Recommendation in Table 1; drugs listed alphabetically. SHD indicates structural heart disease (including ischemic heart disease).
Junctional tachycardia is a rapid, occasionally irregular, narrow-complex tachycardia (with rates typically of 120 bpm to 220 bpm) (Figure 20). AV dissociation (often isorhythmic) may be seen, and when present, excludes the misdiagnosis of AVRT and makes AVNRT highly unlikely. Other SVTs are often misdiagnosed and misclassified as junctional tachycardia because of the frequent absence of demonstrable P waves in reentrant rhythms. Furthermore, when it is irregular, junctional tachycardia may be misdiagnosed as AF or MAT. The mechanism for junctional tachycardia is enhanced (abnormal) automaticity from an ectopic focus in the AV junction (including the His bundle).388
Junctional tachycardia is uncommon in adults388; it is typically seen in infants postoperatively, after cardiac surgery for congenital heart disease; this is also known as junctional ectopic tachycardia. As such, there is limited evidence with regard to diagnosis and management of junctional tachycardia in adult patients. Adults with junctional tachycardia typically have a relatively benign course, whereas infants and children with acquired or congenital junctional tachycardia have a high rate of death due to heart failure or an uncontrollable, incessant tachyarrhythmia.
There are data to support the use of beta blockers, diltiazem, flecainide, procainamide, propafenone, and verapamil for the treatment of junctional tachycardia (see recommendations and references in Sections 8.1 and 8.2). The efficacy of amiodarone has been reported only in pediatric patients.389,390 Digoxin has not been well established as chronic therapy for junctional tachycardia.
A related rhythm, nonparoxysmal junctional tachycardia (more commonly known as accelerated AV junctional rhythm), is far more common in adults than paroxysmal junctional tachycardia. The mechanism of nonparoxysmal junctional tachycardia is associated with automaticity or triggered activity. It occurs at a slower rate (70 bpm to 130 bpm) and is often due to digoxin toxicity391 or myocardial infarction.392,393 Treatment of this rhythm centers on addressing the underlying condition. In addition, there is some evidence that beta blockers,394 intravenous adenosine, or verapamil395 can terminate an accelerated junctional arrhythmia. A transient junction rhythm may be seen after slow-pathway ablation for AVNRT.396
8.1. Acute Treatment: Recommendations
| COR | LOE | Recommendations |
|---|---|---|
| IIa | C-LD | 1. Intravenous beta blockers are reasonable for acute treatment in patients with symptomatic junctional tachycardia.388 |
| See Online Data Supplement 19. | In a series studying junctional tachycardia in adult patients, beta-blocker therapy—specifically intravenous propranolol—was found to be modestly effective in terminating and/or reducing the incidence of tachycardia.388 | |
| IIa | C-LD | 2. Intravenous diltiazem, procainamide, or verapamil is reasonable for acute treatment in patients with junctional tachycardia.397 |
| See Online Data Supplement 19. | There may be a limited role for intravenous diltiazem, procainamide, or verapamil in patients in whom beta blockers are ineffective. The literature supports the use of intravenous verapamil, alone or in combination with procainamide; less is known about diltiazem monotherapy.397 The addition of procainamide to propranolol may be more effective than propranolol monotherapy.388 | |
8.2. Ongoing Management: Recommendations
| COR | LOE | Recommendations |
|---|---|---|
| IIa | C-LD | 1. Oral beta blockers are reasonable for ongoing management in patients with junctional tachycardia.388 |
| See Online Data Supplement 19. | Beta blockers are often used as first-line chronic therapy for junctional tachycardia because of the important proarrhythmic effects and long-term toxicity of other agents that have been shown to be effective.389,398,399 When junctional tachycardia is paroxysmal, attention should be directed toward avoiding the potential for bradyarrhythmias and hypotension when beta-blocker therapy is initiated. | |
| IIa | C-LD | 2. Oral diltiazem or verapamil is reasonable for ongoing management in patients with junctional tachycardia.397 |
| See Online Data Supplement 19. | Junctional tachycardia caused by enhanced automaticity may be suppressed as effectively by diltiazem and verapamil as by beta blockers. In 1 study of an adult patient on a regimen of oral verapamil, procainamide, and digoxin in combination for prophylactic therapy, junctional tachycardia could not be induced by either atrial or ventricular overdrive pacing, programmed electrical stimulation, or isoproterenol.397 Less is known about oral diltiazem than verapamil, but it likely has a similar efficacy. | |
| IIb | C-LD | 1. Flecainide or propafenone may be reasonable for ongoing management in patients without structural heart disease or ischemic heart disease who have junctional tachycardia.398,399 |
| See Online Data Supplement 19. | Studies supporting the use of flecainide for long-term control of junctional tachycardia were performed at a time when intravenous flecainide was given for acute control, followed by a transition to chronic oral therapy.398 Although the only data for propafenone stem from case series in infants and children, given that flecainide and propafenone reduce automaticity from the ectopic focus in the AV junction, either agent is reasonable, provided patients do not have structural heart disease or ischemic heart disease.213,400,401 | |
| IIb | C-LD | 2. Catheter ablation may be reasonable in patients with junctional tachycardia when medical therapy is not effective or contraindicated.131,132,396,402–405 |
| See Online Data Supplement 19. | Radiofrequency ablation has been performed as a potentially curative therapy for junctional tachycardia since the early 1990s. However, in view of the reported 5% to 10% risk of AV block, catheter ablation is generally reserved for highly symptomatic patients in whom drug therapy has been ineffective or not tolerated (Table 8). Many practitioners use cryoablation as an alternative to radiofrequency ablation.132 Given that it is often difficult to distinguish junctional tachycardia from AVNRT on the ECG, EP study with the goal of ablation may be a helpful diagnostic and potentially therapeutic intervention. Junctional tachycardia may be observed during and after slow-pathway ablation of AVNRT, because of irritation of the compact AV node.406 This iatrogenic junctional tachycardia is transient and generally benign and can be distinguished from AVNRT through pacing maneuvers at EP study.396,405 It is crucial to recognize this phenomenon at the time of EP study because attempts to ablate the junctional tachycardia are unnecessary and could result in AV block. | |
9. Special Populations
9.1. Pediatrics
As discussed in the Scope (Section 1.4), the present document is aimed at the adult population (≥18 years of age) and offers no specific recommendations for pediatric patients. Nevertheless, a brief discussion of SVT in pediatric patients is included below, highlighting major considerations with regard to SVT in younger patients, including adolescent patients.
SVT in young patients varies significantly from SVT in adult patients in terms of mechanism, risk of developing heart failure or cardiac arrest, risks associated with interventional therapy, natural history, and psychosocial impact. Approximately half of pediatric SVT presents in the first 4 months of life, with age-related peaks in occurrence subsequently at 5 to 8 years and after 13 years. Accessory pathway–mediated tachycardia accounts for >70% of SVT in infants, decreasing to approximately 55% in adolescents.56,407–409 AVNRT increases with age, from 9% to 13% of SVT in infants, to 30% to 50% of SVT in teenagers. After 12 years of age, women are more likely to have AVNRT than men, and overall SVT is less frequent among African American and Hispanic patients than in the general pediatric population.56 Atrial flutter is seen in some neonates and in older children is predominantly observed after congenital heart disease. AF is uncommon in childhood, accounting for <3% of supraventricular arrhythmias, and may be a consequence of AVRT or AVNRT in adolescents or may be associated with repaired congenital heart disease. Symptoms of SVT vary with age: gastrointestinal or respiratory findings in infants, chest or abdominal pain in the younger child, and palpitations in the adolescent. Congestive heart failure is present in up to 20% of infants and in older children with incessant tachycardia and in rare cases may necessitate mechanical cardiopulmonary support during initial therapy.410
Pre-excitation is present in 20% to 35% of children with SVT. The risk of ventricular fibrillation or SCD related to WPW in childhood is 1.3% to 1.6% and is highest in the first 2 decades of life.60,254–257 The risk of cardiac arrest is higher in patients with AVRT precipitating AF, short accessory connection refractory periods, and posteroseptal accessory pathways.60,254–257 Notably, the absence of prior symptoms does not preclude risk because cardiac arrest may be the initial manifestation of pre-excitation.254,257,411 Risk stratification, such as with ambulatory 24-hour monitoring or treadmill exercise testing, is often considered for children with pre-excitation to assess persistence of pre-excitation.412
Pharmacological therapy of SVT in childhood is largely based on practice patterns because RCTs of antiarrhythmic medications in children are lacking. AV nodal–blocking drugs are widely used for the most common arrhythmias, AVRT, and AVNRT. Higher initial doses of adenosine are needed in children than in adults, with children receiving from 150 mcg/kg to 250 mcg/kg.413–415 Digoxin and propranolol have similar efficacy in infants with SVT without pre-excitation.416 Digoxin is avoided in the presence of pre-excitation because its use in infancy has been associated with SCD or ventricular fibrillation.417,418 Amiodarone, sotalol, propafenone, or flecainide can be used for refractory SVT in infants. In older children presenting with SVT, beta-blocker therapy is most often the initial therapy used. Because of the rare occurrence of adverse events with flecainide, including in patients without structural heart disease, ischemic heart disease, or ventricular dysfunction, flecainide is not used as a first-line medication in children.419
Catheter ablation can be successfully performed in children of all ages, with acute success rates comparable to those reported in adults.281,282,420,421 Success rates are influenced by the presence of structural heart disease or ischemic heart disease and are highest in left-sided accessory pathways and lowest for AT. Complications were reported in 4% to 8% of the initial large series, with major complications in 0.9% to 3.2%, and complication rates were higher in patients weighing <15 kg.281,420–422 The implications of complications, including AV block requiring pacing, perforation, and coronary artery or mitral valve injury, are profound in young patients.423–425 In early series, death was reported in 0.12% of children with normal hearts and was associated with lower weight and increased number of ablation lesions.426 Increased institutional experience, advanced mapping techniques, and use of cryoablation have reduced the incidence of complications, as well as the radiation exposure associated with the procedure. Although most centers perform elective ablation for children weighing >12 kg to 15 kg, ablation in younger or smaller children is generally reserved for those with medically refractory SVT or tachycardia-induced cardiomyopathy or before surgery that may limit access for subsequent catheter-based procedures. Recurrence rates for SVT after successful procedures are higher than reported in large adult series, ranging from 7% to 17%; whether this reflects technical differences, natural history, or more long-term follow-up is unclear.427–429 Recurrence is highest among right-sided accessory pathways, particularly anteroseptal or multiple pathways, and in AT in the setting of complex congenital heart disease.427–429
Junctional ectopic tachycardia occurs predominantly in very young patients either as a congenital form or, more commonly, after intracardiac repair of congenital heart disease. Nonpostoperative junctional tachycardia has been reported to respond to amiodarone or combination therapy including beta blockers, flecainide, procainamide, or propafenone.130 Ablation for patients with refractory tachycardia or ventricular dysfunction has shown efficacy of 82% to 85%, but inadvertent AV block occurred in 18% and recurrence was seen in 14% of patients.130 Postoperative junctional tachycardia occurs in 2% to 10% of young patients undergoing intracardiac surgery, particularly for ventricular or AV septal defects, tetralogy of Fallot, transposition of the great arteries, and Norwood procedures.430,431 Treatment includes sedation with muscle relaxation, limitation of inotropic medications, reduction of core temperature to 34 to 35°C, atrial overdrive pacing, and procainamide or amiodarone infusions.416,432–435 In general, postoperative junctional tachycardia resolves and does not require ongoing therapy.
Although this guideline focuses on adults, it should be noted that SVT may occur in the fetus and, if sustained, may put the fetus at risk of cardiovascular collapse manifested by hydrops. Mothers require safety monitoring by adult cardiologists during treatment. The most common mechanisms for fetal SVT are AVRT and atrial flutter.436 Persistent SVT with hydrops carries a high mortality rate, and therefore, prompt and aggressive treatment is warranted. Maternal administration of antiarrhythmic agents has been shown to be effective through transplacental delivery. Flecainide, sotalol, and digoxin, alone or in combination, have demonstrated arrhythmia termination rates of 60% to 90%, depending on whether hydrops is present.437,438 In cases refractory to the aforementioned drugs, maternal oral loading for 2 to 7 days with amiodarone may prove lifesaving.439 Treatment of fetal SVT has provided safety data for treatment of arrhythmias in women during pregnancy, as addressed in Section 9.3.
9.2. Patients With Adult Congenital Heart Disease
See Figure 22 for the algorithm for acute treatment of non–pre-excited SVT in adult congenital heart disease (ACHD) patients; and Figure 23 for the algorithm for ongoing management of non–pre-excited SVT in ACHD patients.

- Download figure
- Download PowerPoint
Figure 22. Acute treatment of SVT in ACHD patients. Colors correspond to Class of Recommendation in Table 1; drugs listed alphabetically. *For rhythms that break or recur spontaneously, synchronized cardioversion is not appropriate. ACHD indicates adult congenital heart disease; IV, intravenous; and SVT, supraventricular tachycardia.

- Download figure
- Download PowerPoint
Figure 23. Ongoing management of SVT in ACHD patients. Colors correspond to Class of Recommendation in Table 1; drugs listed alphabetically. ACHD indicates adult congenital heart disease; intra-op, intraoperative; pre-op, preoperative; and SVT, supraventricular tachycardia.
9.2.1. Clinical Features
SVT is observed in 10% to 20% of ACHD patients, and is associated with a significantly increased risk of heart failure, stroke, and SCD.440–444 The most common mechanism of SVT in ACHD patients is macroreentrant AT (also called flutter), which accounts for at least 75% of SVT and frequently involves the CTI. Focal AT, AVNRT, and accessory pathway–mediated tachycardia each account for less than about 8% of SVT, whereas the incidence of AF is about 10% and increases with age.133,445–449 AT occurs in 20% to 45% of adults with Ebstein anomaly, single-ventricle/Fontan procedures, tetralogy of Fallot, transposition of the great arteries, and atrial septal defects.449–451
The management of SVT in ACHD patients is influenced by the underlying cardiac anatomy and surgical repair, the current hemodynamic sequelae of the anatomy and repairs, and mechanism of SVT. The ventricular rate during SVT may be slowed because of variable AV conduction, which can result in a delay in recognizing tachycardia and the development of congestive failure. Recognition of severe forms of congenital heart disease, including unrepaired or palliated defects, cyanotic heart disease, single or systemic right ventricles, or Ebstein anomaly, is essential to decision making during SVT treatment. In certain conditions, the presence of cyanosis or severe ventricular dysfunction requires consideration of high-risk cardioversion with resuscitation measures at hand; usually, this decision is made at centers with specialized expertise. Management of ACHD patients should be undertaken only in collaboration with a cardiologist who has specialized training or experience in managing such patients.
RCTs assessing antiarrhythmic medication efficacy are lacking. Beta-blocking medications offer the advantages of outpatient medication initiation and may provide protection from tachycardia-mediated hypotension or ischemia. Risks of proarrhythmia are increased with the use of sotalol, ibutilide, dofetilide, and particularly flecainide and require in-hospital initiation. Flecainide is associated with increased risk of SCD419 and is reserved for patients without ventricular dysfunction who do not respond to other therapy. Sinus node dysfunction may contribute to the development of atrial arrhythmias and may become exacerbated with antiarrhythmic medications. Atrial antibradycardia pacing to maintain a consistent physiological heart rate may decrease the frequency of tachycardia episodes and may improve functional capacity.364,370,371 Atrial antitachycardia pacing to terminate atrial reentry tachycardia is an effective approach when feasible.364,370,371
Overall acute success rates of catheter ablation procedures for SVT in ACHD patients range from 70% to 85%, with recurrences in 20% to 60% of patients within 2 years.452–457 Catheter ablation is challenged by limitations of venous access to the heart, hypertrophied atrial tissue, multiple atrial reentrant circuits, and atrial baffles partitioning the coronary sinus and CTI to the pulmonary venous atrium. Because the CTI is involved in >60% of atrial reentry circuits, an initial strategy targeting this region is often used. Highest success rates are achieved in patients with atrial septal defects, approaching 90% to 100%, although subsequent AF has been reported in 11% to 30% of patients within 3 years.330,449,458 Because of the need for sophisticated knowledge of anatomy, advanced mapping capability, cardiac anesthesia with careful periprocedural monitoring, and repeat ablations, such patients should be referred to centers with extensive experience in complex congenital heart disease ablations.
The development of atrial arrhythmias in ACHD patients is often an indicator of progressive hemodynamic changes, which require in-depth functional and hemodynamic assessment. Intervention for residual hemodynamic/structural defects may need to be planned as part of chronic arrhythmia management. Patients with Ebstein anomaly or repaired tetralogy of Fallot may have significant pulmonary regurgitation, tricuspid regurgitation, or both, which might benefit from reoperation. In some settings, integration of operative ablation techniques with hemodynamic repair may be optimal.
9.2.2. Acute Treatment: Recommendations
| COR | LOE | Recommendations |
|---|---|---|
| I | C-LD | 1. Acute antithrombotic therapy is recommended in ACHD patients who have AT or atrial flutter to align with recommended antithrombotic therapy for patients with AF.365 |
| See Online Data Supplements 16 and 17. | The risk of stroke associated with AF is well established, and several RCTs have demonstrated the efficacy of anticoagulation for stroke prevention in patients with additional risk factors.366 Anticoagulation has also been shown to prevent stroke when administered during the weeks immediately before and after cardioversion.31,367 Whether atrial flutter carries a risk of stroke similar to that in patients with AF has been debated in the past but is now supported by limited evidence from mechanistic, observational, and prospective studies that include patients primarily with atrial flutter.365–369 Several reports have suggested that the risk of stroke in patients with atrial flutter is mitigated by anticoagulation. Meta-analysis of 13 studies of patients undergoing cardioversion of atrial flutter reported short-term stroke risks ranging from 0% to 7%, and the thromboembolism rate in patients with sustained flutter in 4 studies averaged 3% annually.365 Other studies have reported similar risk and effectiveness of anticoagulation.369 ACHD patients with nonfibrillatory atrial tachyarrhythmias appear to be at high risk, as well.367,459 Therefore, on the basis of available data, recommendations for antithrombotic therapy for ACHD patients who also have AT or atrial flutter are similar to those for patients with AF.10 | |
| I | B-NR | 2. Synchronized cardioversion is recommended for acute treatment in ACHD patients and SVT who are hemodynamically unstable.75,460 |
| See Online Data Supplement 20. | A small observational study demonstrated safety and efficacy of synchronized cardioversion in ACHD patients, with sinus rhythm achieved in 94% of patients.460 Guideline-directed medical therapy supports this treatment approach, stressing that electrical cardioversion is the safest and most effective way of treating SVT associated with hemodynamic instability and should be considered early in the management of such patients.75 Modification of electrode pad placement may be necessary, by using an anterior-posterior configuration in patients with marked atrial enlargement or positioning strategically according to the individual arrhythmia and anatomic substrate, including consideration of the possibility of dextrocardia. | |
| I | C-LD | 3. Intravenous diltiazem or esmolol (with extra caution used for either agent, observing for the development of hypotension) is recommended for acute treatment in ACHD patients and SVT who are hemodynamically stable.461,462 |
| See Online Data Supplement 21. | Very limited data exist on the use of calcium channel–blocking or beta-blocking medications for rate control or termination of AT or atrial flutter specifically associated with ACHD. Rate control of AT or atrial flutter with rapid AV conduction may be useful to improve hemodynamic status while planning conversion strategies. Patients with congenital heart disease, particularly single-ventricle physiology or systemic right ventricles, may not tolerate ventricular rates >120 bpm for many hours.29 Either drug may be associated with the development of hypotension in up to 20% of patients.462 Because of the high incidence of ventricular dysfunction in this population, particular attention should be given to monitoring the patient for development of hypotension, which would necessitate prompt dose adjustments or change of treatment strategy. | |
| I | B-NR | 4. Intravenous adenosine is recommended for acute treatment in ACHD patients and SVT.207,463–465 |
| See Online Data Supplement 20. | Observational studies support the use of adenosine to terminate AVNRT, SVT using an accessory pathway, and some forms of focal AT. These mechanisms account for <25% of SVT in adults with repaired congenital disease.207,463 Intravenous adenosine is unlikely to terminate atrial reentry tachycardia or atrial flutter, which represents >70% of SVT episodes in this population, but it may be diagnostic by producing transient AV block, which would make the atrial activity visible.464,465 | |
| IIa | B-NR | 1. Intravenous ibutilide or procainamide can be effective for acute treatment in ACHD patients and atrial flutter who are hemodynamically stable.466–468 |
| See Online Data Supplement 20. | A small observational study reported that intravenous ibutilide was successful in conversion of 71% of acute episodes of atrial flutter in ACHD patients; torsades de pointes or nonsustained VT was reported in 2.7% of episodes.468 Pretreatment with magnesium may reduce the incidence of postibutilide ventricular arrhythmias. Small studies support the use of intravenous procainamide as adjunctive therapy for acute therapy of atrial flutter because it improves efficacy of pacing conversion of atrial flutter.466,467 | |
| IIa | B-NR | 2. Atrial pacing can be effective for acute treatment in ACHD patients and SVT who are hemodynamically stable and anticoagulated as per current guidelines for antithrombotic therapy in patients with AF.466,469–472 |
| See Online Data Supplement 20. | Small observational studies support the efficacy of atrial pacing to successfully terminate 54% to 82% of acute episodes of AT or atrial flutter.466,469–472 Atrial pacing is an effective alternative, particularly when concerns about the use of antiarrhythmic medications or significant sinus node dysfunction exist. | |
| IIa | B-NR | 3. Elective synchronized cardioversion can be useful for acute termination of AT or atrial flutter in ACHD patients when acute pharmacological therapy is ineffective or contraindicated.460 |
| See Online Data Supplement 20. | ACHD patients are at increased risk of congestive heart failure and/or atrial thrombus formation with prolonged episodes of AT or atrial flutter. Pharmacological conversion in patients with complex anatomy, ventricular dysfunction, or prolonged QTc intervals may result in significant proarrhythmia or acute hemodynamic deterioration. Early assessment for cardiac thrombus with transesophageal echocardiogram followed by synchronized cardioversion may be preferable to prolonged or multiple attempts to achieve pharmacological cardioversion. Because of frequent coexistent sinus node dysfunction and ventricular dysfunction, the need for chronotropic or inotropic intervention should be anticipated, with appropriate personnel and support present. Modification of electrode pad placement may be necessary, by using an anterior-posterior configuration in patients with marked atrial enlargement or positioning strategically according to the individual arrhythmia and anatomic substrate, including consideration of the possibility of dextrocardia. | |
| IIb | B-NR | 1. Oral dofetilide or sotalol may be reasonable for acute treatment in ACHD patients and AT and/or atrial flutter who are hemodynamically stable.473,474 |
| See Online Data Supplement 20. | Small observational studies support the use of oral dofetilide474 or sotalol473 to acutely convert AT to sinus rhythm in 70% to 85% of episodes of acute AT or atrial flutter. Proarrhythmic events are more commonly reported with dofetilide. One small study of 20 patients reported that 10% experienced torsades de pointes after dofetilide474; both proarrhythmic events occurred in patients with single-ventricle physiology. The risk of proarrhythmia or worsening ventricular function requires arrhythmia monitoring with use. | |
9.2.3. Ongoing Management: Recommendations
| COR | LOE | Recommendations |
|---|---|---|
| I | C-LD | 1. Ongoing management with antithrombotic therapy is recommended in ACHD patients and AT or atrial flutter to align with recommended antithrombotic therapy for patients with AF.365 |
| See Online Data Supplements 16 and 17. | The risk of stroke associated with AF is well established, and several RCTs have demonstrated the efficacy of anticoagulation for stroke prevention in patients with additional risk factors.366 Anticoagulation has also been shown to prevent stroke when administered during the weeks immediately before and after cardioversion.31,367 Whether atrial flutter carries a risk of stroke similar to that in patients with AF has been debated in the past but is now supported by limited evidence from mechanistic, observational, and prospective studies that include patients primarily with atrial flutter.365–369 Several reports have suggested that the risk of stroke in patients with atrial flutter is mitigated by anticoagulation. Meta-analysis of 13 studies of patients undergoing cardioversion of atrial flutter reported short-term stroke risks ranging from 0% to 7%, and the thromboembolism rate in patients with sustained flutter in 4 studies averaged 3% annually.365 Other studies have reported similar risk and effectiveness of anticoagulation.369 ACHD patients with nonfibrillatory atrial tachyarrhythmias appear to be at high risk, as well.367,459 Therefore, on the basis of available data, recommendations for antithrombotic therapy for ACHD patients who also have AT or atrial flutter are similar to those for patients with AF.10 | |
| I | C-LD | 2. Assessment of associated hemodynamic abnormalities for potential repair of structural defects is recommended in ACHD patients as part of therapy for SVT.25,29 |
| See Online Data Supplement 20. | The development of AT, atrial flutter, or AF in ACHD patients is often associated with progressive hemodynamic deterioration of the underlying disease. Surgical treatment of the hemodynamic problems does not eliminate atrial arrhythmias, and ablation of atrial arrhythmias alone could allow significant hemodynamic issues to progress and potentially deteriorate. Successful treatment involves assessing both the arrhythmia and the contributing hemodynamic changes and addressing both when indicated and feasible. Early experience in adults with unoperated atrial septal defects and atrial arrhythmias demonstrated the importance of an integrated approach for arrhythmia and hemodynamic problems; similar principles apply to patients with tetralogy of Fallot, Ebstein anomaly, and single-ventricle physiology; these patients are at highest risk of arrhythmia development, with concurrent hemodynamic abnormalities. For example, patients with ASD who underwent surgery before the age of 25 years had better long-term outcomes and a lower incidence of atrial arrhythmias than those who underwent surgery later in life.475 Closure of significant atrial septal defects in adults even later in life improves morbidity and survival476 but is associated with new (7%) or recurrent (60%) AT.475 Arrhythmias still can be treated successfully when they develop later after surgical corrections; catheter ablation of atrial arrhythmias associated with ASD repair is highly successful, with acute success rates reported as 93% to 100%.449,458,477 Therefore, patients with unoperated significant ASD and arrhythmias should undergo ablation of the AT, as well as closure of the ASD. The choice of catheter versus surgical approaches to therapy is determined by anatomic features of the ASD likely to be successfully addressed with a catheter approach. No randomized comparison trials of catheter versus surgical closure of ASD combined with arrhythmia intervention have been reported. Surgical closure of large ASDs combined with arrhythmia surgery for AT or fibrillation can be safely performed, with 6.5% occurrence of AF reported during 2 years of follow-up.478 | |
| IIa | B-NR | 1. Preoperative catheter ablation or intraoperative surgical ablation of accessory pathways or AT is reasonable in patients with SVT who are undergoing surgical repair of Ebstein anomaly.479–485 |
| See Online Data Supplement 20. | The prevalence of SVT among patients with Ebstein anomaly was 33% in 1 large series, the highest noted among ACHD patients,440 and increased with age. AT, atrial flutter, or AF develops in ≥50% of patients with Ebstein anomaly and significant tricuspid regurgitation. Right-sided accessory pathways are present in 15% to 30% of patients with Ebstein anomaly and may be multiple in up to ≥29% of those patients. Catheter ablation of accessory pathways in patients with Ebstein anomaly is associated with lower success rates than for other populations, acute success rate of 75% to 89% of procedures, with acute recurrence in 25% to 30%.480,481 In a series of 83 adults undergoing arrhythmia surgery at the time of surgical repair of Ebstein anomaly, accessory pathways were present in 32%, and atrial flutter/fibrillation was noted in 54%,483 with no recurrence of AP after surgery. Successful surgical ablation of accessory pathways has been reported in 92% to 100%.483,484 In a series of patients undergoing right atrial maze procedures or isthmus ablation for atrial flutter/fibrillation in association with repair of Ebstein anomaly, freedom from recurrent flutter/fibrillation was 75% during 34 months of follow-up.483 In a comparison study of combined operative arrhythmia surgery with repair, versus catheter ablation followed by surgical repair, the combined approach was effective in 94% of cases versus 76% of patients treated with the catheter approach alone.484 In a series of patients undergoing repair of Ebstein anomaly, patients who underwent preoperative EP study with intraoperative ablation of arrhythmia substrate had lower risk of SCD than patients without arrhythmia intervention.479 In patients with Ebstein anomaly undergoing planned surgical intervention, an integrated approach of arrhythmia intervention has been demonstrated to be safe and effective. | |
| IIa | B-NR | 2. Oral beta blockers or sotalol therapy can be useful for prevention of recurrent AT or atrial flutter in ACHD patients.218,447,486 |
| See Online Data Supplement 20. | Beta blockers may decrease catecholamine-related triggers and provide AV nodal blockade during recurrent atrial arrhythmias. One study of adults with transposition of the great arteries and prior atrial switch repairs with implanted defibrillators demonstrated SVT preceding VT in 50% of patients; use of beta-blocker medications in this population was associated with decreased incidence of appropriate defibrillator shocks.486 Observational studies on the use of sotalol in ACHD patients report freedom from recurrent AT in 41% to 46% of patients during short-term follow-up.218,487,488 Use of either medication in the setting of significant sinus node dysfunction may exacerbate bradycardia and requires careful monitoring. Initiation of sotalol in this population is recommended during inpatient monitoring for proarrhythmia for 48 to 72 hours. | |
| IIa | B-NR | 3. Catheter ablation is reasonable for treatment of recurrent symptomatic SVT in ACHD patients.330,449,452,454,457,458,489–492 |
| See Online Data Supplement 20. | Multiple observational and multicenter studies have demon strated acute success rates between 65% and 100% for treatment of SVT associated with ACHD.452,454,455,457,489,493,494 Acute success rates vary by tachycardia mechanism and type of congenital heart disease and repair. Success rates are highest for SVT associated with AVNRT (>80%),490,494 accessory pathways (75% to 89% among patients with Ebstein anomaly),480 or focal AT (80% to 100%).492,494,495 Success rates for catheter ablation of AT or atrial flutter are significantly lower than that reported in patients without ACHD, with overall 65% to 82% acute success in mixed anatomic substrates,452,454,455,457,493,494 but success rates have improved with advanced mapping and ablation techniques.494 Acute success rates in ablation of AT or atrial flutter varies significantly by type of congenital heart disease, ranging from 93% to 100% in patients with repaired ASD,330,449,458 85% to 100% in atrial baffle repairs of transposition of the great arteries,490,492 and 54% to 75% of univentricular heart or Fontan repairs.457,490,495 Older age and presence of univentricular heart physiology was associated with decreased acute success rates in a series of 193 ablations of AT.457 Recurrent AT has been reported in 20% to 85% of patients during short-term follow-up, with development of AF reported in 7% to 30% of patients.330,449,454,493 Recurrent SVT may represent the same or a new reentrant circuit, and arrhythmia burden may be decreased by ablation procedures. Ablation procedures in patients with complex congenital heart disease are best performed in centers with advanced mapping techniques and expertise in congenital heart disease.25,29,496 | |
| IIa | B-NR | 4. Surgical ablation of AT or atrial flutter can be effective in ACHD patients undergoing planned surgical repair.497–508 |
| See Online Data Supplement 20. | In patients with symptomatic SVT undergoing planned surgical repairs of structural heart disease or ischemic heart disease, observation studies report that arrhythmia surgery can be integrated into the operation with high efficacy and without increased surgical morbidity.497–500 In populations including those with tetralogy of Fallot/double-outlet right ventricle, tricuspid valve repairs, and ASD, rates of freedom from recurrent AT or atrial flutter have been reported as 73% to 93% without antiarrhythmic medications during medium-term follow-up of 2.5 to 10 years. Catheter ablation may be attempted in specialized centersbefore surgical Fontan repair. There are no RCTs comparing efficacy of catheter versus surgical ablation of AT or atrial flutter in patients with prior Fontan repairs. The incidence of AT or atrial flutter in patients with prior Fontan repairs approaches 60% and increases with advancing age. Catheter ablation success rates in this subset of patients are lower than for other lesions,457 with acute success in 54% to 75%, and with recurrent AT in 27% to 50% of patients within 2 to 4 years490,495; death or transplantation was reported in 27% during follow-up. Several observational studies of surgical resection and ablation for AT or atrial flutter associated with Fontan conversion reported rates of freedom from AT of 12% to 16% during follow-up extending to 8 years501–505; death or transplantation was reported in 6% to 14% during follow-up. Performance of a modified right atrial maze procedure versus CTI ablation was associated with lower recurrence rates.501 Alternatively, surgical repairs without arrhythmia intervention are associated with high recurrence rates of AT. Arrhythmia surgery can be combined with hemodynamic surgical revision with low mortality rate and improved medium-term freedom from arrhythmia recurrence and deathin this population. | |
| IIb | B-NR | 1. Atrial pacing may be reasonable to decrease recurrences of AT or atrial flutter in ACHD patients and sinus node dysfunction.472,509,510 |
| See Online Data Supplement 20. | Small observational studies support the use of atrial pacing in patients with recurrent AT or atrial flutter to reduce the frequency of tachycardia episodes. The pacing rate is programmed to maintain atrial pacing for the majority of the time in patients with sinus node dysfunction, significantly reducing tachycardia recurrences to 11% in 1 study.509,510 In addition, the implanted pacemaker can be used for termination of recurrent episodes of AT or atrial flutter.472 | |
| IIb | B-NR | 2. Oral dofetilide may be reasonable for prevention of recurrent AT or atrial flutter in ACHD patients.447,474,487,488 |
| See Online Data Supplement 20. | Two small observational studies on the use of oral dofetilide for ACHD patients report a high rate of acute conversion of AT or atrial flutter, with longer-term efficacy of the drug (defined by either complete suppression or partial improvement of symptoms) ranging from 70% to 85%. The benefit was tempered by an association with torsades de pointes in 10%.471,484 Although many patients continued to experience recurrence, these episodes were better tolerated and were of limited duration.471,484 Because of the potential risk of proarrhythmia, dofetilide is usually a second-line medication after failure of beta blockers and sotalol. Initiation of oral dofetilide is recommended during 72-hour inpatient monitoring. | |
| IIb | B-NR | 3. Amiodarone may be reasonable for prevention of recurrent AT or atrial flutter in ACHD patients for whom other medications and catheter ablation are ineffective or contraindicated.447 |
| See Online Data Supplement 20. | An observational study reported efficacy of amiodarone in maintaining sinus rhythm, although side effects were noted frequently, including thyroid disorders or AV block.447 The risk of significant side effects increases with chronic use and with higher dosages; using the minimal effective chronic dosage is recommended. Observational studies reported thyroid disorders in 13% to 36% of ACHD patients receiving amiodarone; risk factors for thyrotoxicosis include female sex, cyanotic heart disease, low body mass index, prior Fontan procedure, or dosages >200 mg daily.511,512 Amiodarone is recommended for short-term use or for patients for whom alternative therapy is not an option for prevention of recurrent AT. | |
| III: Harm | B-NR | 1. Flecainide should not be administered for treatment of SVT in ACHD patients and significant ventricular dysfunction.419 |
| See Online Data Supplement 20. | There are no RCTs on the use of flecainide in ACHD patients and ventricular dysfunction. One retrospective study collected data on 579 young patients, 369 of whom received flecainide for the treatment of SVT. Efficacy for the treatment of SVT was 71%. Among 12 patients with cardiac arrest while receiving flecainide for SVT, 8 had congenital heart disease, and 7 of 8 had either mild to moderate ventricular dysfunction or systemic right- or single-ventricle anatomy. The risk associated with flecainide treatment of SVT associated with congenital heart disease appears to be highest among patients with complex heart disease or ventricular dysfunction. | |
9.3. Pregnancy
Pregnancy may confer an increased susceptibility to a variety of arrhythmias, even in the absence of underlying heart disease.513 Pregnancy is also associated with an increased risk of arrhythmia exacerbation, such as more frequent and refractory tachycardia episodes, in patients with a pre-existing arrhythmic substrate.514 An important consideration is that adverse maternal and fetal outcomes have been reported as a result of SVT in pregnancy.515 Although there is potential toxicity to the fetus with certain pharmacological and nonpharmacological therapies, safe options exist to allow for treating most cases of maternal SVT effectively.
The literature on therapeutic options for the management of arrhythmias in pregnancy is generally limited to single case reports or small series and favors the use of older antiarrhythmic agents because of more abundant reports on the safe use of these drugs. Experience with use of drugs in pregnancy also comes from treating a variety of maternal and fetal conditions, not maternal SVT alone. Although all medications have potential side effects to both the mother and the fetus at any stage of pregnancy, if possible, drugs should be avoided in the first trimester, when risk of congenital malformations is greatest. The lowest recommended dose should be used initially, accompanied by regular monitoring of clinical response.
9.3.1. Acute Treatment: Recommendations
| COR | LOE | Recommendations |
|---|---|---|
| I | C-LD | 1. Vagal maneuvers are recommended for acute treatment in pregnant patients with SVT.235,516 |
| See Online Data Supplement 20. | For acute conversion of SVT, vagal maneuvers, including Valsalva and carotid sinus massage, can be performed quickly and should be the first-line intervention to terminate SVT. These maneuvers should be performed with the patient in the supine position. Vagal maneuvers typically will not be effective if the rhythm does not involve the AV node as a requisite component of a reentrant circuit. There is no "gold standard" for proper Valsalva maneuver technique, but in general, the patient raises intrathoracic pressure by bearing down against a closed glottis for 10 to 30 seconds, equivalent to at least 30 mm Hg to 40 mm Hg.82,84 Carotid massage is performed after absence of bruit has been confirmed by auscultation, by applying steady pressure over the right or left carotid sinus for 5 to 10 seconds.83,84 Another vagal maneuver based on the classic diving reflex consists of applying an ice-cold, wet towel to the face85; in a laboratory setting, facial immersion in water at 10°C (50°F) has proved effective in terminating tachycardia, as well.86 One study involving 148 nonpregnant patients with SVT demonstrated that Valsalva was more successful than carotid sinus massage, and switching from 1 technique to the other resulted in an overall success rate of 27.7%.82 The practice of applying pressure to the eyeball is potentially dangerous and has been abandoned. | |
| I | C-LD | 2. Adenosine is recommended for acute treatment in pregnant patients with SVT.516 |
| See Online Data Supplement 20. | When vagal maneuvers fail to terminate SVT, adenosine is a first-line drug option for pregnant patients.516 Adverse effects to the fetus would not be expected, given that it is unlikely the drug will reach the fetal circulation because of adenosine's short half-life.517 Maternal side effects, such as chest discomfort and flushing, are usually transient. The initial dose for adenosine is 6-mg rapid bolus IV. If this is ineffective, up to 2 subsequent infusions of 12 mg may be administered. Higher doses of adenosine may be necessary in some cases; safe administration of up to 24 mg has been reported.518 | |
| I | C-LD | 3. Synchronized cardioversion is recommended for acute treatment in pregnant patients with hemodynamically unstable SVT when pharmacological therapy is ineffective or contraindicated.516 |
| See Online Data Supplement 20. | Synchronized cardioversion has been reported to be safe at all stages of pregnancy. The electrode pads should be applied such that the energy source and trajectory are directed away from the uterus.519 Fetal monitoring during cardioversion (if time allows) and during the immediate postcardioversion period is recommended.519 Energy dosing for pregnant patients should be the same as in nonpregnant patients.520 | |
| IIa | C-LD | 1. Intravenous metoprolol or propranolol is reasonable for acute treatment in pregnant patients with SVT when adenosine is ineffective or contraindicated.516 |
| See Online Data Supplement 20. | Beta-adrenergic–blocking drugs are considered the first-line option for a variety of arrhythmias in pregnancy because there are extensive reports on their safe use from treating a variety of maternal conditions over many decades. A slow infusion would be less likely to cause hypotension.516,517,521 | |
| IIb | C-LD | 1. Intravenous verapamil may be reasonable for acute treatment in pregnant patients with SVT when adenosine and beta blockers are ineffective or contraindicated.516 |
| See Online Data Supplement 20. | Intravenous verapamil has been used effectively for the acute treatment of SVT in pregnant women; however, there is a higher risk of maternal hypotension with intravenous verapamil than with adenosine.516 Reports on diltiazem use for acute SVT termination in pregnancy are more limited than for verapamil, yet similar effects are expected.517 | |
| IIb | C-LD | 2. Intravenous procainamide may be reasonable for acute treatment in pregnant patients with SVT.522 |
| See Online Data Supplement 20. | Intravenous procainamide has been used safely to treat a variety of maternal and fetal supraventricular and ventricular arrhythmias and can be effective when used for acute conversion.517,522 Procainamide is generally best avoided as long-term therapy because it can cause a lupus-like syndrome, unless other options are contraindicated or ineffective. | |
| IIb | C-LD | 3. Intravenous amiodarone may be considered for acute treatment in pregnant patients with potentially life-threatening SVT when other therapies are ineffective or contraindicated.517,523 |
| See Online Data Supplement 20. | Although intravenous amiodarone has been administered safely during pregnancy, multiple adverse effects to the fetus have also been reported.523 An important concern is the possibility of fetal hypothyroidism, reported in approximately 17% of cases.517,523 With an intravenous infusion for short-term use, side effects would be less of a concern, given that most toxicities are related to cumulative drug dose. | |
9.3.2. Ongoing Management: Recommendations
| COR | LOE | Recommendations |
|---|---|---|
| IIa | C-LD | 1. The following drugs, alone or in combination, can be effective for ongoing management in pregnant patients with highly symptomatic SVT: |
| a. Digoxin437,517 | ||
| b. Flecainide437,517 | ||
| c. Metoprolol517,521 | ||
| d. Propafenone517 | ||
| e. Propranolol517,521 | ||
| f. Sotalol437,517 | ||
| g. Verapamil517 | ||
| See Online Data Supplement 20. | If possible, antiarrhythmic drugs should be avoided in the first trimester, when risk of congenital malformations is greatest. Almost no reports exist on the use of newer antiarrhythmic drugs (such as dofetilide) during pregnancy, yet their use may be justified if the benefits outweigh the risk. The lowest recommended dose should be used initially, with dose adjustments made according to clinical response. For oral chronic prophylaxis, drugs such as metoprolol, propranolol, and digoxin are considered safe first-line agents because of their longer record of safety, yet caution is advised, given that therapy with beta blockers has been associated with intrauterine growth retardation.517,524 This effect appears to be especially pronounced with atenolol, especially in mothers who received atenolol earlier in gestational age and who were treated for longer duration.525 Flecainide and propafenone have been used effectively to treat a variety of maternal and fetal tachycardias, yet they are reserved for patients who have no underlying structural heart disease or ischemic heart disease.113 | |
| IIb | C-LD | 1. Catheter ablation may be reasonable in pregnant patients with highly symptomatic, recurrent, drug-refractory SVT with efforts toward minimizing radiation exposure.526,527 |
| See Online Data Supplement 20. | The risk of radiation exposure to the fetus is a concern with catheter ablation in pregnant patients, because high-dose ionizing radiation has been linked to excess malignancy and congenital malformations.528 However, the fetal radiation dose for most common cardiovascular interventions is not likely to exceed the 50-mGy negligible-risk threshold dose for excess malignancy.529 One study that used phantoms to simulate pregnancy estimated a low lifetime risk of malignancies from radiation exposure to the conceptus during a typical ablation procedure.527 Furthermore, with current technologies such as electroanatomic mapping systems, catheter ablation procedures using minimal or even zero fluoroscopy have been described in pregnant women.526 Thus, if a catheter ablation procedure is required in a pregnant woman, radiation-reduction technologies should be used, and the procedure should be avoided in the first trimester when the teratogenic risk is greatest.528 Of note, shielding the fetus by covering the mother with a lead apron does not eliminate radiation to the fetus because most of the radiation to the fetus comes from scatter. | |
| IIb | C-LD | 2. Oral amiodarone may be considered for ongoing management in pregnant patients when treatment of highly symptomatic, recurrent SVT is required and other therapies are ineffective or contraindicated.517,523 |
| See Online Data Supplement 20. | Although oral amiodarone has been administered safely during pregnancy, multiple adverse effects to the fetus have also been reported.523 An important concern is the possibility of fetal hypothyroidism, reported in approximately 17% of cases.517,523 Therefore, fetal monitoring for development of goiter with ultrasound and for signs of clinical hypothyroidism is advised. In addition, amiodarone has the potential for direct neurotoxicity, which may lead to neurodevelopmental abnormalities.523 | |
9.4. SVT in Older Populations
9.4.1. Acute Treatment and Ongoing Management: Recommendation
The natural history of SVT is steadily changing because most patients with SVT undergo ablation at a younger age, but in general, the relative proportion of AT is higher in older populations, and AVNRT is more prevalent than AVRT among patients undergoing ablation.49 Atypical atrial flutter and macroreentrant AT are on the rise as consequences of increasing AF ablation in this patient population, yet there are limited outcome data from RCTs for this segment of the population. Therapeutic decisions should be balanced between the overall risks and benefits of the invasive nature of ablation versus long-term commitment to pharmacological therapy.
| COR | LOE | Recommendation |
|---|---|---|
| I | B-NR | 1. Diagnostic and therapeutic approaches to SVT should be individualized in patients more than 75 years of age to incorporate age, comorbid illness, physical and cognitive functions, patient preferences, and severity of symptoms.66,67,530–538 |
| See Online Data Supplement 20. | Data have consistently demonstrated that ablation is highly successful (>95%) in selected older patients.66,67,531–536 Outcomes from 48 medical centers in Germany were reported among 3234 consecutive patients undergoing AVNRT ablation from 2007 to 2010; of the total, 259 patients (8%) were >75 years of age.537 Acute success was achieved in 98.5% of the older patient group, similar to the 2 younger patient groups (98.7% for the group <50 years of age; 98.8% for the group 50 to 75 years of age). In this study, complication rates were low; hemodynamically stable pericardial effusion was observed in 2 of 259 patients (0.8%), and no pacemakers were needed in the older patient group. Similarly, additional studies from older patient cohorts have consistently shown that older patients have more comorbid medical conditions, have a higher incidence of structural heart disease or ischemic heart disease, and have more severe symptoms associated with SVT.530,538,539 A few studies have shown that complications may be slightly higher in older patients than in younger patients, although the overall complications are low and acceptable.530,538,539 These ablation outcome data should be balanced with the risks and benefits of pharmacological therapy when therapeutic options are reviewed with older patients. | |
10. Quality-of-Life Considerations
Patients with SVT may experience recurring symptoms that negatively impact their quality of life. Episodes of tachycardia can cause lightheadedness and syncope, which can become an obstacle to the performance of usual activities of daily living (eg, driving).72 However, there are minimal data on the effect of treatment on the quality of life for patients with SVT. In 1 study that evaluated patients with SVT who underwent ablation or received medical therapy, questionnaires such as the 36-Item Short-Form Health Survey revealed improved quality-of-life scores in several categories, including physical role functioning (perceived disability from physical limitations), general health perceptions (perceived physical and mental health), and emotional role functioning (perceived disability from emotional limitations).540 These improvements, measured at 1 to 5 years of follow-up, were greater in patients who underwent ablation than in those treated with medical therapy. Other literature, using domains from the 36-Item Short-Form Health Survey541–543 and other quality- of-life questionnaires,544–546 suggests that quality of life is improved after ablation for PSVT. However, these data carry important limitations, particularly a lack of an appropriate control group, small sample sizes, and referral bias. Furthermore, patients affected by PSVT carry different experiences. Therefore, firm conclusions cannot be drawn about the effect on quality of life provided by medical or ablation therapy, and no recommendations are provided.
See Online Data Supplement 22 for additional data on quality-of-life considerations.
11. Cost-Effectiveness
Given the rising costs of health care, there is a growing enthusiasm for incorporating economic appraisals of available therapies and resources into guidelines. The "2014 ACC/AHA Statement on Cost/Value Methodology in Clinical Practice Guidelines and Performance Measures"6 called for development of Level of Value categories to accompany COR and LOE in future guidelines. Although basing recommendations on a cost-effectiveness approach to therapy is an important goal for the current and future healthcare economy, it also poses considerable challenges. For example, the cost of therapy, available technology, and practice patterns are highly dynamic, and there may be some cost associated with unintended harm or complications that result from any intervention. Furthermore, the approach toward evaluating the burden of cost in the literature is based on varied perspectives (eg, individual, third party, stakeholder, societal).
The small body of literature evaluating cost-effectiveness strategies in PSVT has traditionally centered on an evaluation of medical therapy versus catheter ablation. A rigorous cost-effectiveness Markov model was conducted in 2000 to compare radiofrequency ablation to medical management with generic metoprolol from the societal perspective.105 The estimated population consisted of patients with AVNRT (approximately 65%) and AVRT. On the basis of this simulation, the authors concluded that, for symptomatic patients with monthly episodes of PSVT, radiofrequency ablation was the more effective and less expensive strategy when compared with medical therapy. An observational cohort study of patients with atrial flutter supported early ablation to significantly reduce hospital-based healthcare utilization and the risk of AF.547
These studies, along with other older literature, favor catheter ablation over medical therapy as the more cost-effective approach to treating PSVT and atrial flutter. However, the results of these studies were based on cost data and practice patterns that do not apply to the current environment and practice. Therefore, no recommendations are provided.
See Online Data Supplement 23 for additional data on cost-effectiveness.
12. Shared Decision Making
It is important that the patient be included in clinical decision-making processes, with consideration of his/her preferences and goals for therapy, as well as his/her unique physical, psychological, and social situation. In selected cases, personalized, self-directed interventions can be developed in partnership with the patient, such as vagal maneuvers and "pill-in-the-pocket" drug therapy.
Shared decision making is especially important for patients with SVT. As seen in this guideline, SVT treatment can be nuanced and requires expert knowledge of EP processes and treatment options. Treatment options are highly specific to the exact type of arrhythmia and can depend on certain characteristics of a particular arrhythmia (eg, whether there is pre-excitation). The various choices for therapy, including drugs, cardioversion, invasive treatment, or a combination thereof, can be confusing to the patient, so a detailed explanation of the benefits and risks must be included in the conversation.
Patients are encouraged to ask questions with time allotted for caregivers to respond. Providing a relaxed atmosphere, anticipating patient concerns, and encouraging patients to keep a notebook with questions could facilitate productive conversations.
It is also important that clinicians use lay terminology to explain treatment options to their patients. This involves a clear explanation of the risks and benefits of each recommendation, including how other comorbidities may impact each treatment option. Discussions with other physicians and healthcare providers caring for the patient will provide the broadest picture available. A full discussion about decisions for subsequent care and any further instructions is important to reinforce these issues before the patient leaves the healthcare setting. It is the responsibility of the physician and healthcare team to provide the patient with the best possible understanding of all management options in terms of risks, benefits, and potential effects on quality of life.
13. Evidence Gaps and Future Research Needs
SVTs, even with the exclusion of AF, are among the most common arrhythmias that require medical intervention. The decade before the publication of the "2003 ACC/AHA/ESC Guidelines for the Management of Patients With Supraventricular Arrhythmias"11 was characterized by major shifts in understanding of the mechanism for SVT, as well as a sea change in management (because of catheter ablation). Since the early 2000s, there have been many iterative but important advances in pharmacological and invasive management for SVT. Catheter ablation is even better established, with a high degree of success and low complication rate, especially for the most common types of SVT, such as AVNRT and AVRT. Drug options, on the other hand, are relatively unchanged since publication of the 2003 guideline, perhaps relating to ongoing concern about potential adverse side effects of antiarrhythmic agents.
Areas of uncertainty remain, including interventions for which advanced technology is less important. For example, vagal maneuvers are recommended in many circumstances as first-line intervention in patients with SVT, but they are often ineffective. Furthermore, there is great variation in the way these maneuvers are administered. Therefore, research on the best technique of vagal maneuvers, with dissemination of the findings, is necessary. Clinical trials on antiarrhythmic drugs for SVT have been limited, and data are often extrapolated from studies that primarily focused on management of patients with AF. The efficacy of a variety of drugs is likely to differ according to the tachycardia mechanism, and therefore differentiating the best drug for each individual arrhythmia is necessary; for example, the efficacy of class III agents might be markedly different in patients with AF than in patients with atrial flutter. Limited data exist on the optimal management of less common types of SVTs, such as junctional tachycardia and multifocal AT. Therefore, in view of significant gaps that remain with regard to optimal management of patients with SVT, we must consider the role of electronic medical records, registries, and national datasets to better acquire observational data when trials are not available or feasible. Multicenter registry studies would allow for expansion of our knowledge on the best pharmacological and nonpharmacological approaches to treat these arrhythmias. In collaboration with national societies, the National Institutes of Health, and the US Food and Drug Administration, registries could be developed across selected centers to gather important information on safety and long-term outcomes where data are lacking (just as such registries are being developed for AF ablation). Mandatory postmarket surveillance data collection on new drugs for SVT could also be considered by the US Food and Drug Administration as a condition for drug approval.
The mechanism and primary etiology of IST remains to be defined—advances here would provide a first step on finding better therapies for this disorder. It should be noted that medical advances have resulted in an increase in the number of patients with SVT in specific populations, such as in patients after ablation (especially AF), ACHD patients, and patients of advanced age. As the numbers of these often-complicated patients grow, opportunities arise to perform clinical research to guide future recommendations.
New pharmacological therapies are needed, especially for SVT in patients for whom ablation is not an option or has been unsuccessful. Newer drugs that selectively target atrial channels currently under investigation for patients with AF should be investigated for management of AT. Both mapping and ablation techniques need to be further investigated to maximize the likelihood of successful ablation with minimal risk. In the outpatient setting, the added value of new personal monitoring and implantable devices needs to be assessed, and studies of the impact of shared decision making with patients on outcomes are needed for personal monitoring innovations. Finally, we encourage investigation of quality-of-life improvement strategies, in addition to cost-effectiveness studies, for the management of SVT.
Presidents and Staff
American College of Cardiology
Kim A. Williams, Sr, MD, FACC, FAHA, President
Shalom Jacobovitz, Chief Executive Officer
William J. Oetgen, MD, MBA, FACC, Executive Vice President, Science, Education, and Quality
Amelia Scholtz, PhD, Publications Manager, Science, Education, and Quality
American College of Cardiology/American Heart Association
Lisa Bradfield, CAE, Director, Guidelines and Clinical Policy
Abdul R. Abdullah, MD, Associate Science and Medicine Advisor
Alexa Papaila, Project Manager, Science and Clinical Policy
American Heart Association
Mark A. Creager, MD, FACC, FAHA, President
Nancy Brown, Chief Executive Officer
Rose Marie Robertson, MD, FAHA, Chief Science Officer
Gayle R. Whitman, PhD, RN, FAHA, FAAN, Senior Vice President, Office of Science Operations
Marco Di Buono, PhD, Vice President, Science, Research, and Professional Education
Jody Hundley, Production Manager, Scientific Publications, Office of Science Operations
Acknowledgments
We thank Dr. Daniel B. Mark for his invaluable assistance in reviewing studies relating to quality of life and cost-effectiveness. His research and insight informed much of the discussion on these topics. We also thank Dr. Sarah A. Spinler for her contributions with regard to antiarrhythmic drug therapy.
Footnotes
This document was approved by the American College of Cardiology Board of Trustees and Executive Committee, the American Heart Association Science Advisory and Coordinating Committee, and the Heart Rhythm Society Board of Trustees in August 2015 and the American Heart Association Executive Committee in September 2015.
The Author Comprehensive Relationships Data Supplement is available with this article at http://circ.ahajournals.org/lookup/suppl/doi:10.1161/CIR.0000000000000311/-/DC1.
The Data Supplement files are available with this article at http://circ.ahajournals.org/lookup/suppl/doi:10.1161/CIR.0000000000000311/-/DC2.
The American Heart Association requests that this document be cited as follows: Page RL, Joglar JA, Caldwell MA, Calkins H, Conti JB, Deal BJ, Estes NAM 3rd, Field ME, Goldberger ZD, Hammill SC, Indik JH, Lindsay BD, Olshansky B, Russo AM, Shen W-K, Tracy CM, Al-Khatib SM. 2015 ACC/AHA/HRS guideline for the management of adult patients with supraventricular tachycardia: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2016;133;e506-e574. doi: 10.1161/CIR.0000000000000311.
This article has been copublished in the Journal of the American College of Cardiology and Heart Rhythm Journal.
Copies: This document is available on the World Wide Web sites of the American College of Cardiology (www.acc.org), the American Heart Association (professional.heart.org), and the Heart Rhythm Society (www.hrsonline.org). A copy of the document is available at http://professional.heart.org/statements by using either "Search for Guidelines & Statements" or the "Browse By Topic" area. To purchase additional reprints, call 843-216-2533 or e-mail [email protected].
Expert peer review of AHA Scientific Statements is conducted by the AHA Office of Science Operations. For more on AHA statements and guidelines development, visit http://professional.heart.org/statements. Select the "Guidelines & Statements" drop-down menu, then click "Publication Development."
Permissions: Multiple copies, modification, alteration, enhancement, and/or distribution of this document are not permitted without the express permission of the American Heart Association. Instructions for obtaining permission are located at http://www.heart.org/HEARTORG/General/Copyright-Permission-Guidelines_UCM_300404_Article.jsp. A link to the "Copyright Permissions Request Form" appears on the right side of the page.
References
- 1. Committee on Standards for Developing Trustworthy Clinical Practice Guidelines, Institute of Medicine (US). Clinical Practice Guidelines We Can Trust . Washington, DC: National Academies Press, 2011.Google Scholar
- 2. Committee on Standards for Systematic Reviews of Comparative Effectiveness Research, Institute of Medicine (US). Finding What Works in Health Care: Standards for Systematic Reviews . Washington, DC: National Academies Press, 2011.Google Scholar
- 3. ACCF/AHA Task Force on Practice Guidelines. Methodology Manual and Policies From the ACCF/AHA Task Force on Practice Guidelines . American College of Cardiology and American Heart Association. 2010. Available at: http://assets.cardiosource.com/Methodology_Manual_for_ACC_AHA_Writing_Committees.pdf and http://my.americanheart.org/idc/groups/ahamah-public/@wcm/@sop/documents/downloadable/ucm_319826.pdf. Accessed January 23, 2015.Google Scholar
- 4.
Jacobs AK, Kushner FG, Ettinger SM, et al. . ACCF/AHA clinical practice guideline methodology summit report: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation . 2013; 127:268–310.LinkGoogle Scholar - 5.
Jacobs AK, Anderson JL, Halperin JL . The evolution and future of ACC/AHA clinical practice guidelines: a 30-year journey: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation . 2014; 130:1208–17.LinkGoogle Scholar - 6.
Anderson JL, Heidenreich PA, Barnett PG, et al. . ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. Circulation . 2014; 129:2329–45.LinkGoogle Scholar - 7.
Halperin JL, Levine GN, Al-Khatib SM . Further evolution of the ACC/AHA clinical practice guideline recommendation classification system: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Clinical Practice Guidelines. Circulation . 2016; 133:1426–28.LinkGoogle Scholar - 8.
Arnett DK, Goodman RA, Halperin JL, et al. . AHA/ACC/HHS strategies to enhance application of clinical practice guidelines in patients with cardiovascular disease and comorbid conditions: from the American Heart Association, American College of Cardiology, and US Department of Health and Human Services. Circulation . 2014; 130:1662–7.LinkGoogle Scholar - 9.
Al-Khatib SM, Arshad A, Balk EM, et al. . Risk stratification for arrhythmic events in patients with asymptomatic pre-excitation: a systematic review for the 2015 ACC/AHA/HRS guideline for the management of adult patients with supraventricular tachycardia: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation . 2016; 133:e575–86.LinkGoogle Scholar - 10.
January CT, Wann LS, Alpert JS, et al. . 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation 2014; 130:e199–267.LinkGoogle Scholar - 11.
Blömstrom-Lundqvist C, Scheinman MM, Aliot EM, et al. . ACC/AHA/ESC guidelines for the management of patients with supraventricular arrhythmias—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Supraventricular Arrhythmias). Developed in collaboration with NASPE-Heart Rhythm Society. Circulation . 2003; 108:1871–1909.LinkGoogle Scholar - 12.
Fihn SD, Blankenship JC, Alexander KP, et al. . 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation . 2014; 130:1749–67.LinkGoogle Scholar - 13.
Fihn SD, Gardin JM, Abrams J, et al. . 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation . 2012; 126:e354–471.LinkGoogle Scholar - 14.
Nishimura RA, Otto CM, Bonow RO, et al. . 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation . 2014; 129:e521–643.LinkGoogle Scholar - 15.
Goff DC, Lloyd-Jones DM, Bennett G, et al. . 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation . 2014; 129[suppl 2]:S49–73.LinkGoogle Scholar - 16.
Yancy CW, Jessup M, Bozkurt B, et al. . 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation . 2013; 128:e240–327.LinkGoogle Scholar - 17.
Whitlock RP, Sun JC, Fremes SE, et al. . Antithrombotic and thrombolytic therapy for valvular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest . 2012; 141:e576S–600S.CrossrefMedlineGoogle Scholar - 18.
Camm AJ, Lip GYH, De Caterina R, et al. . 2012 Focused update of the ESC guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J . 2012; 33:2719–47.CrossrefMedlineGoogle Scholar - 19.
European Heart Rhythm AssociationEuropean Association for Cardio-Thoracic Surgery ,Camm AJ, et al. . Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J . 2010; 31:2369–429.CrossrefMedlineGoogle Scholar - 20.
Epstein AE, DiMarco JP, Ellenbogen KA, et al. . 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation . 2013; 127:e283–352.LinkGoogle Scholar - 21.
Verma A, Cairns JA, Mitchell LB, et al. . 2014 Focused update of the Canadian Cardiovascular Society guidelines for the management of atrial fibrillation. Can J Cardiol . 2014; 30:1114–30.CrossrefMedlineGoogle Scholar - 22.
Cairns JA, Connolly S, McMurtry S, et al. . Canadian Cardiovascular Society atrial fibrillation guidelines 2010: prevention of stroke and systemic thromboembolism in atrial fibrillation and flutter. Can J Cardiol . 2011; 27:74–90.CrossrefMedlineGoogle Scholar - 23.
Gersh BJ, Maron BJ, Bonow RO, et al. . 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Developed in collaboration with the American Association for Thoracic Surgery, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation . 2011; 124:e783–831.LinkGoogle Scholar - 24.
Smith SC, Benjamin EJ, Bonow RO, et al. . AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation . 2011; 124:2458–73.LinkGoogle Scholar - 25.
Warnes CA, Williams RG, Bashore TM, et al. . ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines on the Management of Adults With Congenital Heart Disease). Developed in Collaboration With the American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation . 2008; 118:e714–833.LinkGoogle Scholar - 26.
Chobanian AV, Bakris GL, Black HR, et al. . the National High Blood Pressure Education Program Coordinating Committee: seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension . 2003; 42:1206–52.LinkGoogle Scholar - 27. PACES/HRS expert consensus statement on the use of catheter ablation in children and patients with congenital heart disease. J Am Coll Cardiol . 2015; IN PRESS.Google Scholar
- 28.
Sheldon RS, Grubb BP, Olshansky B, et al. . 2015 Heart Rhythm Society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm . 2015; 12:e41–63.CrossrefMedlineGoogle Scholar - 29.
Khairy P, Van Hare GF, Balaji S, et al. . PACES/HRS expert consensus statement on the recognition and management of arrhythmias in adult congenital heart disease. Developed in partnership between the Pediatric and Congenital Electrophysiology Society (PACES) and the Heart Rhythm Society (HRS). Heart Rhythm . 2014; 11:e102–65.CrossrefMedlineGoogle Scholar - 30.
Calkins H, Kuck KH, Cappato R, et al. . 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. J Interv Card Electrophysiol . 2012; 33:171–257.CrossrefMedlineGoogle Scholar - 31.
Field JM, Hazinski MF, Sayre MR, et al. . Part 1: executive summary: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation . 2010; 122:S640–56.LinkGoogle Scholar - 32.
Orejarena LA, Vidaillet H, DeStefano F, et al. . Paroxysmal supraventricular tachycardia in the general population. J Am Coll Cardiol . 1998; 31:150–7.CrossrefMedlineGoogle Scholar - 33.
Calkins H, Sousa J, el-Atassi R, et al. . Diagnosis and cure of the Wolff-Parkinson-White syndrome or paroxysmal supraventricular tachycardias during a single electrophysiologic test. N Engl J Med . 1991; 324:1612–8.CrossrefMedlineGoogle Scholar - 34.
Kay GN, Epstein AE, Dailey SM, et al. . Role of radiofrequency ablation in the management of supraventricular arrhythmias: experience in 760 consecutive patients. J Cardiovasc Electrophysiol . 1993; 4:371–89.CrossrefMedlineGoogle Scholar - 35.
Lindsay BD, Chung MK, Gamache MC, et al. . Therapeutic end points for the treatment of atrioventricular node reentrant tachycardia by catheter-guided radiofrequency current. J Am Coll Cardiol . 1993; 22:733–40.CrossrefMedlineGoogle Scholar - 36.
Jackman WM, Beckman KJ, McClelland JH, et al. . Treatment of supraventricular tachycardia due to atrioventricular nodal reentry, by radiofrequency catheter ablation of slow-pathway conduction. N Engl J Med . 1992; 327:313–8.CrossrefMedlineGoogle Scholar - 37.
Chen SA, Chiang CE, Yang CJ, et al. . Accessory pathway and atrioventricular node reentrant tachycardia in elderly patients: clinical features, electrophysiologic characteristics and results of radiofrequency ablation. J Am Coll Cardiol . 1994; 23:702–8.CrossrefMedlineGoogle Scholar - 38.
Rodriguez LM, de Chillou C, Schläpfer J, et al. . Age at onset and gender of patients with different types of supraventricular tachycardias. Am J Cardiol . 1992; 70:1213–5.CrossrefMedlineGoogle Scholar - 39.
Wu D, Yeh SJ, Wang CC, et al. . A simple technique for selective radiofrequency ablation of the slow pathway in atrioventricular node reentrant tachycardia. J Am Coll Cardiol . 1993; 21:1612–21.CrossrefMedlineGoogle Scholar - 40.
Clair WK, Wilkinson WE, McCarthy EA, et al. . Spontaneous occurrence of symptomatic paroxysmal atrial fibrillation and paroxysmal supraventricular tachycardia in untreated patients. Circulation . 1993; 87:1114–22.CrossrefMedlineGoogle Scholar - 41.
Wu D, Denes P, Amat-y-Leon F, et al. . Clinical, electrocardiographic and electrophysiologic observations in patients with paroxysmal supraventricular tachycardia. Am J Cardiol . 1978; 41:1045–51.CrossrefMedlineGoogle Scholar - 42.
DiMarco JP, Miles W, Akhtar M, et al. . Adenosine for paroxysmal supraventricular tachycardia: dose ranging and comparison with verapamil. Assessment in placebo-controlled, multicenter trials. The Adenosine for PSVT Study Group. Ann Intern Med . 1990; 113:104–10.CrossrefMedlineGoogle Scholar - 43.
Bhandari AK, Anderson JL, Gilbert EM, et al. . Correlation of symptoms with occurrence of paroxysmal supraventricular tachycardia or atrial fibrillation: a transtelephonic monitoring study. The Flecainide Supraventricular Tachycardia Study Group. Am Heart J . 1992; 124:381–6.CrossrefMedlineGoogle Scholar - 44.
Sintetos AL, Roark SF, Smith MS, et al. . Incidence of symptomatic tachycardia in untreated patients with paroxysmal supraventricular tachycardia. Arch Intern Med . 1986; 146:2205–9.CrossrefMedlineGoogle Scholar - 45.
Henthorn RW, Waldo AL, Anderson JL, et al. . Flecainide acetate prevents recurrence of symptomatic paroxysmal supraventricular tachycardia. The Flecainide Supraventricular Tachycardia Study Group. Circulation . 1991; 83:119–25.LinkGoogle Scholar - 46.
Dorian P, Naccarelli GV, Coumel P, et al. . A randomized comparison of flecainide versus verapamil in paroxysmal supraventricular tachycardia. The Flecainide Multicenter Investigators Group. Am J Cardiol . 1996; 77:89A–95A.CrossrefMedlineGoogle Scholar - 47.
Lu C-W, Wu M-H, Chen H-C, et al. . Epidemiological profile of Wolff-Parkinson-White syndrome in a general population younger than 50 years of age in an era of radiofrequency catheter ablation. Int J Cardiol . 2014; 174:530–4.CrossrefMedlineGoogle Scholar - 48.
Whinnett ZI, Sohaib SMA, Davies DW . Diagnosis and management of supraventricular tachycardia. BMJ . 2012; 345:e7769.CrossrefMedlineGoogle Scholar - 49.
Porter MJ, Morton JB, Denman R, et al. . Influence of age and gender on the mechanism of supraventricular tachycardia. Heart Rhythm . 2004; 1:393–6.CrossrefMedlineGoogle Scholar - 50.
Campbell RW . Supraventricular tachycardia: occasional nuisance or frequent threat? Eur Heart J . 1996; 17Suppl C:21–5.CrossrefMedlineGoogle Scholar - 51.
Cairns CB, Niemann JT . Intravenous adenosine in the emergency department management of paroxysmal supraventricular tachycardia. Ann Emerg Med . 1991; 20:717–21.CrossrefMedlineGoogle Scholar - 52.
Goyal R, Zivin A, Souza J, et al. . Comparison of the ages of tachycardia onset in patients with atrioventricular nodal reentrant tachycardia and accessory pathway-mediated tachycardia. Am Heart J . 1996; 132:765–7.CrossrefMedlineGoogle Scholar - 53.
Maryniak A, Bielawska A, Bieganowska K, et al. . Does atrioventricular reentry tachycardia (AVRT) or atrioventricular nodal reentry tachycardia (AVNRT) in children affect their cognitive and emotional development? Pediatr Cardiol . 2013; 34:893–7.CrossrefMedlineGoogle Scholar - 54.
González-Torrecilla E, Almendral J, Arenal A, et al. . Combined evaluation of bedside clinical variables and the electrocardiogram for the differential diagnosis of paroxysmal atrioventricular reciprocating tachycardias in patients without pre-excitation. J Am Coll Cardiol . 2009; 53:2353–8.CrossrefMedlineGoogle Scholar - 55.
Liu S, Yuan S, Hertervig E, et al. . Gender and atrioventricular conduction properties of patients with symptomatic atrioventricular nodal reentrant tachycardia and Wolff-Parkinson-White syndrome. J Electrocardiol . 2001; 34:295–301.CrossrefMedlineGoogle Scholar - 56.
Anand RG, Rosenthal GL, Van Hare GF, et al. . Is the mechanism of supraventricular tachycardia in pediatrics influenced by age, gender or ethnicity? Congenit Heart Dis . 2009; 4:464–8.CrossrefMedlineGoogle Scholar - 57.
Erdogan A, Schulte B, Carlsson J, et al. . [Clinical characteristics of patients with AV-nodal reentrant tachycardia (AVNRT): modification by high frequency catheter ablation. Study of 748 patients after high frequency catheter ablation]. Med Klin (Munich) . 2001; 96:708–12.CrossrefMedlineGoogle Scholar - 58.
Lee SH, Chen SA, Wu TJ, et al. . Effects of pregnancy on first onset and symptoms of paroxysmal supraventricular tachycardia. Am J Cardiol . 1995; 76:675–8.CrossrefMedlineGoogle Scholar - 59.
Walfridsson U, Strömberg A, Janzon M, et al. . Wolff-Parkinson-White syndrome and atrioventricular nodal re-entry tachycardia in a Swedish population: consequences on health-related quality of life. Pacing Clin Electrophysiol . 2009; 32:1299–306.CrossrefMedlineGoogle Scholar - 60.
Cain N, Irving C, Webber S, et al. . Natural history of Wolff-Parkinson-White syndrome diagnosed in childhood. Am J Cardiol . 2013; 112:961–5.CrossrefMedlineGoogle Scholar - 61.
Lessmeier TJ, Gamperling D, Johnson-Liddon V, et al. . Unrecognized paroxysmal supraventricular tachycardia: potential for misdiagnosis as panic disorder. Arch Intern Med . 1997; 157:537–43.CrossrefMedlineGoogle Scholar - 62.
Laurent G, Leong-Poi H, Mangat I, et al. . Influence of ventriculoatrial timing on hemodynamics and symptoms during supraventricular tachycardia. J Cardiovasc Electrophysiol . 2009; 20:176–81.CrossrefMedlineGoogle Scholar - 63.
Abe H, Nagatomo T, Kobayashi H, et al. . Neurohumoral and hemodynamic mechanisms of diuresis during atrioventricular nodal reentrant tachycardia. Pacing Clin Electrophysiol . 1997; 20:2783–8.CrossrefMedlineGoogle Scholar - 64.
Auricchio A, Klein H, Trappe HJ, et al. . Lack of prognostic value of syncope in patients with Wolff-Parkinson-White syndrome. J Am Coll Cardiol . 1991; 17:152–8.CrossrefMedlineGoogle Scholar - 65.
Drago F, Turchetta A, Calzolari A, et al. . Reciprocating supraventricular tachycardia in children: low rate at rest as a major factor related to propensity to syncope during exercise. Am Heart J . 1996; 132:280–5.CrossrefMedlineGoogle Scholar - 66.
Kalusche D, Ott P, Arentz T, et al. . AV nodal re-entry tachycardia in elderly patients: clinical presentation and results of radiofrequency catheter ablation therapy. Coron Artery Dis . 1998; 9:359–63.CrossrefMedlineGoogle Scholar - 67.
Haghjoo M, Arya A, Heidari A, et al. . Electrophysiologic characteristics and results of radiofrequency catheter ablation in elderly patients with atrioventricular nodal reentrant tachycardia. J Electrocardiol . 2007; 40:208–13.CrossrefMedlineGoogle Scholar - 68.
Leitch JW, Klein GJ, Yee R, et al. . Syncope associated with supraventricular tachycardia: an expression of tachycardia rate or vasomotor response? Circulation . 1992; 85:1064–71.CrossrefMedlineGoogle Scholar - 69.
Razavi M, Luria DM, Jahangir A, et al. . Acute blood pressure changes after the onset of atrioventricular nodal reentrant tachycardia: a time-course analysis. J Cardiovasc Electrophysiol . 2005; 16:1037–40.CrossrefMedlineGoogle Scholar - 70.
Sganzerla P, Fabbiocchi F, Grazi S, et al. . Electrophysiologic and haemodynamic correlates in supraventricular tachycardia. Eur Heart J . 1989; 10:32–9.CrossrefMedlineGoogle Scholar - 71.
Hamdan MH, Zagrodzky JD, Page RL, et al. . Effect of P-wave timing during supraventricular tachycardia on the hemodynamic and sympathetic neural response. Circulation . 2001; 103:96–101.CrossrefMedlineGoogle Scholar - 72.
Walfridsson U, Walfridsson H . The impact of supraventricular tachycardias on driving ability in patients referred for radiofrequency catheter ablation. Pacing Clin Electrophysiol . 2005; 28:191–5.CrossrefMedlineGoogle Scholar - 73.
Brugada P, Brugada J, Mont L, et al. . A new approach to the differential diagnosis of a regular tachycardia with a wide QRS complex. Circulation . 1991; 83:1649–59.CrossrefMedlineGoogle Scholar - 74.
Vereckei A, Duray G, Szénási G, et al. . New algorithm using only lead aVR for differential diagnosis of wide QRS complex tachycardia. Heart Rhythm . 2008; 5:89–98.CrossrefMedlineGoogle Scholar - 75.
Neumar RW, Otto CW, Link MS, et al. . Part 8: adult advanced cardiovascular life support: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation . 2010; 122:S729–67.LinkGoogle Scholar - 76.
Knight BP, Zivin A, Souza J, et al. . Use of adenosine in patients hospitalized in a university medical center. Am J Med . 1998; 105:275–80.CrossrefMedlineGoogle Scholar - 77.
Willems S, Shenasa M, Borggrefe M, et al. . Atrioventricular nodal reentry tachycardia: electrophysiologic comparisons in patients with and without 2:1 infra-His block. Clin Cardiol . 1993; 16:883–8.CrossrefMedlineGoogle Scholar - 78.
Pava LF, Perafán P, Badiel M, et al. . R-wave peak time at DII: a new criterion for differentiating between wide complex QRS tachycardias. Heart Rhythm . 2010; 7:922–6.CrossrefMedlineGoogle Scholar - 79.
Letsas KP, Weber R, Siklody CH, et al. . Electrocardiographic differentiation of common type atrioventricular nodal reentrant tachycardia from atrioventricular reciprocating tachycardia via a concealed accessory pathway. Acta Cardiol . 2010; 65:171–6.CrossrefMedlineGoogle Scholar - 80.
Knight BP, Ebinger M, Oral H, et al. . Diagnostic value of tachycardia features and pacing maneuvers during paroxysmal supraventricular tachycardia. J Am Coll Cardiol . 2000; 36:574–82.CrossrefMedlineGoogle Scholar - 81.
Murman DH, McDonald AJ, Pelletier AJ, et al. . US emergency department visits for supraventricular tachycardia, 1993–2003. Acad Emerg Med . 2007; 14:578–81.MedlineGoogle Scholar - 82.
Lim SH, Anantharaman V, Teo WS, et al. . Comparison of treatment of supraventricular tachycardia by Valsalva maneuver and carotid sinus massage. Ann Emerg Med . 1998; 31:30–5.CrossrefGoogle Scholar - 83.
Luber S, Brady WJ, Joyce T, et al. . Paroxysmal supraventricular tachycardia: outcome after ED care. Am J Emerg Med . 2001; 19:40–2.CrossrefMedlineGoogle Scholar - 84.
Waxman MB, Wald RW, Sharma AD, et al. . Vagal techniques for termination of paroxysmal supraventricular tachycardia. Am J Cardiol . 1980; 46:655–64.CrossrefMedlineGoogle Scholar - 85.
Tavşanoglu S, Ozenel E . Ice-water washcloth rather than facial immersion (diving reflex) for supraventricular tachycardia in adults. Am J Cardiol . 1985; 56:1003.CrossrefMedlineGoogle Scholar - 86.
Wayne MA . Conversion of paroxysmal atrial tachycardia by facial immersion in ice water. JACEP . 1976; 5:434–5.CrossrefMedlineGoogle Scholar - 87.
Brady WJ, DeBehnke DJ, Wickman LL, et al. . Treatment of out-of-hospital supraventricular tachycardia: adenosine vs verapamil. Acad Emerg Med . 1996; 3:574–85.CrossrefMedlineGoogle Scholar - 88.
Gausche M, Persse DE, Sugarman T, et al. . Adenosine for the prehospital treatment of paroxysmal supraventricular tachycardia. Ann Emerg Med . 1994; 24:183–9.CrossrefMedlineGoogle Scholar - 89.
Madsen CD, Pointer JE, Lynch TG . A comparison of adenosine and verapamil for the treatment of supraventricular tachycardia in the prehospital setting. Ann Emerg Med . 1995; 25:649–55.CrossrefMedlineGoogle Scholar - 90.
McCabe JL, Adhar GC, Menegazzi JJ, et al. . Intravenous adenosine in the prehospital treatment of paroxysmal supraventricular tachycardia. Ann Emerg Med . 1992; 21:358–61.CrossrefMedlineGoogle Scholar - 91.
Rankin AC, Oldroyd KG, Chong E, et al. . Value and limitations of adenosine in the diagnosis and treatment of narrow and broad complex tachycardias. Br Heart J . 1989; 62:195–203.CrossrefMedlineGoogle Scholar - 92.
Lim SH, Anantharaman V, Teo WS, et al. . Slow infusion of calcium channel blockers compared with intravenous adenosine in the emergency treatment of supraventricular tachycardia. Resuscitation . 2009; 80:523–8.CrossrefMedlineGoogle Scholar - 93. Adenoscan IV (adenosine injection) for rapid bolus intravenous use. US Food and Drug Administration. 2015. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/019937s026lbl.pdf. Accessed July 29, 2015.Google Scholar
- 94.
Roth A, Elkayam I, Shapira I, et al. . Effectiveness of prehospital synchronous direct-current cardioversion for supraventricular tachyarrhythmias causing unstable hemodynamic states. Am J Cardiol . 2003; 91:489–91.CrossrefMedlineGoogle Scholar - 95.
Stec S, Kryñski T, Kułakowski P . Efficacy of low energy rectilinear biphasic cardioversion for regular atrial tachyarrhythmias. Cardiol J . 2011; 18:33–8.MedlineGoogle Scholar - 96.
Gupta A, Naik A, Vora A, et al. . Comparison of efficacy of intravenous diltiazem and esmolol in terminating supraventricular tachycardia. J Assoc Physicians India . 1999; 47:969–72.MedlineGoogle Scholar - 97.
Lim SH, Anantharaman V, Teo WS . Slow-infusion of calcium channel blockers in the emergency management of supraventricular tachycardia. Resuscitation . 2002; 52:167–74.CrossrefMedlineGoogle Scholar - 98.
Mauritson DR, Winniford MD, Walker WS, et al. . Oral verapamil for paroxysmal supraventricular tachycardia: a long-term, double-blind randomized trial. Ann Intern Med . 1982; 96:409–12.CrossrefMedlineGoogle Scholar - 99.
Winniford MD, Fulton KL, Hillis LD . Long-term therapy of paroxysmal supraventricular tachycardia: a randomized, double-blind comparison of digoxin, propranolol and verapamil. Am J Cardiol . 1984; 54:1138–9.CrossrefMedlineGoogle Scholar - 100.
Hindricks G . The Multicentre European Radiofrequency Survey (MERFS): complications of radiofrequency catheter ablation of arrhythmias. The Multicentre European Radiofrequency Survey (MERFS) Investigators of the Working Group on Arrhythmias of the European Society of Cardiology. Eur Heart J . 1993; 14:1644–53.CrossrefMedlineGoogle Scholar - 101.
Hindricks G . Incidence of complete atrioventricular block following attempted radiofrequency catheter modification of the atrioventricular node in 880 patients. Results of the Multicenter European Radiofrequency Survey (MERFS) The Working Group on Arrhythmias of the European Society of Cardiology. Eur Heart J . 1996; 17:82–8.CrossrefMedlineGoogle Scholar - 102.
Spector P, Reynolds MR, Calkins H, et al. . Meta-analysis of ablation of atrial flutter and supraventricular tachycardia. Am J Cardiol . 2009; 104:671–7.CrossrefMedlineGoogle Scholar - 103.
Calkins H, Yong P, Miller JM, et al. . Catheter ablation of accessory pathways, atrioventricular nodal reentrant tachycardia, and the atrioventricular junction: final results of a prospective, multicenter clinical trial. The Atakr Multicenter Investigators Group. Circulation . 1999; 99:262–70.CrossrefMedlineGoogle Scholar - 104.
Scheinman MM, Huang S . The 1998 NASPE prospective catheter ablation registry. Pacing Clin Electrophysiol . 2000; 23:1020–8.CrossrefMedlineGoogle Scholar - 105.
Cheng CH, Sanders GD, Hlatky MA, et al. . Cost-effectiveness of radiofrequency ablation for supraventricular tachycardia. Ann Intern Med . 2000; 133:864–76.CrossrefMedlineGoogle Scholar - 106.
Bohnen M, Stevenson WG, Tedrow UB, et al. . Incidence and predictors of major complications from contemporary catheter ablation to treat cardiac arrhythmias. Heart Rhythm . 2011; 8:1661–6.CrossrefMedlineGoogle Scholar - 107.
Tendera M, Wnuk-Wojnar AM, Kulakowski P, et al. . Efficacy and safety of dofetilide in the prevention of symptomatic episodes of paroxysmal supraventricular tachycardia: a 6-month double-blind comparison with propafenone and placebo. Am Heart J . 2001; 142:93–8.CrossrefMedlineGoogle Scholar - 108. A randomized, placebo-controlled trial of propafenone in the prophylaxis of paroxysmal supraventricular tachycardia and paroxysmal atrial fibrillation. UK Propafenone PSVT Study Group. Circulation . 1995; 92:2550–7.MedlineGoogle Scholar
- 109.
Chimienti M, Cullen MT, Casadei G . Safety of flecainide versus propafenone for the long-term management of symptomatic paroxysmal supraventricular tachyarrhythmias. Report from the Flecainide and Propafenone Italian Study (FAPIS) Group. Eur Heart J . 1995; 16:1943–51.CrossrefMedlineGoogle Scholar - 110.
Anderson JL, Platt ML, Guarnieri T, et al. . Flecainide acetate for paroxysmal supraventricular tachyarrhythmias. The Flecainide Supraventricular Tachycardia Study Group. Am J Cardiol . 1994; 74:578–84.CrossrefMedlineGoogle Scholar - 111.
Pritchett EL, DaTorre SD, Platt ML, et al. . Flecainide acetate treatment of paroxysmal supraventricular tachycardia and paroxysmal atrial fibrillation: dose-response studies. The Flecainide Supraventricular Tachycardia Study Group. J Am Coll Cardiol . 1991; 17:297–303.CrossrefMedlineGoogle Scholar - 112.
Pritchett EL, McCarthy EA, Wilkinson WE . Propafenone treatment of symptomatic paroxysmal supraventricular arrhythmias. A randomized, placebo-controlled, crossover trial in patients tolerating oral therapy. Ann Intern Med . 1991; 114:539–44.CrossrefMedlineGoogle Scholar - 113.
Echt DS, Liebson PR, Mitchell LB, et al. . Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med . 1991; 324:781–8.CrossrefMedlineGoogle Scholar - 114.
Wanless RS, Anderson K, Joy M, et al. . Multicenter comparative study of the efficacy and safety of sotalol in the prophylactic treatment of patients with paroxysmal supraventricular tachyarrhythmias. Am Heart J . 1997; 133:441–6.CrossrefMedlineGoogle Scholar - 115.
Gambhir DS, Bhargava M, Nair M, et al. . Comparison of electrophysiologic effects and efficacy of single-dose intravenous and long-term oral amiodarone therapy in patients with AV nodal reentrant tachycardia. Indian Heart J . 1996; 48:133–7.MedlineGoogle Scholar - 116.
Rathore SS, Curtis JP, Wang Y, et al. . Association of serum digoxin concentration and outcomes in patients with heart failure. JAMA . 2003; 289:871–8.CrossrefMedlineGoogle Scholar - 117.
Domanovits H, Laske H, Stark G, et al. . Adenosine for the management of patients with tachycardias-a new protocol. Eur Heart J . 1994; 15:589–93.CrossrefMedlineGoogle Scholar - 118.
Turakhia MP, Santangeli P, Winkelmayer WC, et al. . Increased mortality associated with digoxin in contemporary patients with atrial fibrillation: findings from the TREAT-AF study. J Am Coll Cardiol . 2014; 64:660–8.CrossrefMedlineGoogle Scholar - 119.
Steinwender C, Hönig S, Kypta A, et al. . Pre-injection of magnesium sulfate enhances the efficacy of ibutilide for the conversion of typical but not of atypical persistent atrial flutter. Int J Cardiol . 2010; 141:260–5.CrossrefMedlineGoogle Scholar - 120.
Tercius AJ, Kluger J, Coleman CI, et al. . Intravenous magnesium sulfate enhances the ability of intravenous ibutilide to successfully convert atrial fibrillation or flutter. Pacing Clin Electrophysiol . 2007; 30:1331–5.CrossrefMedlineGoogle Scholar - 121.
Pérez FJ, Schubert CM, Parvez B, et al. . Long-term outcomes after catheter ablation of cavo-tricuspid isthmus dependent atrial flutter: a meta-analysis. Circ Arrhythm Electrophysiol . 2009; 2:393–401.LinkGoogle Scholar - 122.
de Loma-Osorio F, Diaz-Infante E, et al. . Spanish Catheter Ablation Registry. 12th Official Report of the Spanish Society of Cardiology Working Group on Electrophysiology and Arrhythmias (2012). Rev Esp Cardiol (Engl Ed) . 2013; 66:983–92.MedlineGoogle Scholar - 123.
Chen SA, Chiang CE, Yang CJ, et al. . Sustained atrial tachycardia in adult patients. Electrophysiological characteristics, pharmacological response, possible mechanisms, and effects of radiofrequency ablation. Circulation . 1994; 90:1262–78.CrossrefMedlineGoogle Scholar - 124.
Biviano AB, Bain W, Whang W, et al. . Focal left atrial tachycardias not associated with prior catheter ablation for atrial fibrillation: clinical and electrophysiological characteristics. Pacing Clin Electrophysiol . 2012; 35:17–27.CrossrefMedlineGoogle Scholar - 125.
Kalman JM, Olgin JE, Karch MR, et al. . "Cristal tachycardias": origin of right atrial tachycardias from the crista terminalis identified by intracardiac echocardiography. J Am Coll Cardiol . 1998; 31:451–9.CrossrefMedlineGoogle Scholar - 126.
Kistler PM, Sanders P, Fynn SP, et al. . Electrophysiological and electrocardiographic characteristics of focal atrial tachycardia originating from the pulmonary veins: acute and long-term outcomes of radiofrequency ablation. Circulation . 2003; 108:1968–75.LinkGoogle Scholar - 127.
Kistler PM, Fynn SP, Haqqani H, et al. . Focal atrial tachycardia from the ostium of the coronary sinus: electrocardiographic and electrophysiological characterization and radiofrequency ablation. J Am Coll Cardiol . 2005; 45:1488–93.CrossrefMedlineGoogle Scholar - 128.
Natale A, Breeding L, Tomassoni G, et al. . Ablation of right and left ectopic atrial tachycardias using a three-dimensional nonfluoroscopic mapping system. Am J Cardiol . 1998; 82:989–92.CrossrefMedlineGoogle Scholar - 129.
Goldberger J, Kall J, Ehlert F, et al. . Effectiveness of radiofrequency catheter ablation for treatment of atrial tachycardia. Am J Cardiol . 1993; 72:787–93.CrossrefMedlineGoogle Scholar - 130.
Collins KK, Van Hare GF, Kertesz NJ, et al. . Pediatric nonpost-operative junctional ectopic tachycardia medical management and interventional therapies. J Am Coll Cardiol . 2009; 53:690–7.CrossrefMedlineGoogle Scholar - 131.
Hamdan M, Van Hare GF, Fisher W, et al. . Selective catheter ablation of the tachycardia focus in patients with nonreentrant junctional tachycardia. Am J Cardiol . 1996; 78:1292–7.CrossrefMedlineGoogle Scholar - 132.
Law IH, Von Bergen NH, Gingerich JC, et al. . Transcatheter cryothermal ablation of junctional ectopic tachycardia in the normal heart. Heart Rhythm . 2006; 3:903–7.CrossrefMedlineGoogle Scholar - 133.
Akar JG, Kok LC, Haines DE, et al. . Coexistence of type I atrial flutter and intra-atrial re-entrant tachycardia in patients with surgically corrected congenital heart disease. J Am Coll Cardiol . 2001; 38:377–84.CrossrefMedlineGoogle Scholar - 134.
Jaïs P, Shah DC, Haïssaguerre M, et al. . Mapping and ablation of left atrial flutters. Circulation . 2000; 101:2928–34.CrossrefMedlineGoogle Scholar - 135.
Coffey JO, d'Avila A, Dukkipati S, et al. . Catheter ablation of scar-related atypical atrial flutter. Europace . 2013; 15:414–9.CrossrefMedlineGoogle Scholar - 136.
Triedman JK, Bergau DM, Saul JP, et al. . Efficacy of radiofrequency ablation for control of intraatrial reentrant tachycardia in patients with congenital heart disease. J Am Coll Cardiol . 1997; 30:1032–8.CrossrefMedlineGoogle Scholar - 137.
Nakagawa H, Shah N, Matsudaira K, et al. . Characterization of reentrant circuit in macroreentrant right atrial tachycardia after surgical repair of congenital heart disease: isolated channels between scars allow "focal" ablation. Circulation . 2001; 103:699–709.CrossrefMedlineGoogle Scholar - 138.
Chan DP, Van Hare GF, Mackall JA, et al. . Importance of atrial flutter isthmus in postoperative intra-atrial reentrant tachycardia. Circulation . 2000; 102:1283–9.CrossrefMedlineGoogle Scholar - 139.
Stevenson IH, Kistler PM, Spence SJ, et al. . Scar-related right atrial macroreentrant tachycardia in patients without prior atrial surgery: electroanatomic characterization and ablation outcome. Heart Rhythm . 2005; 2:594–601.CrossrefMedlineGoogle Scholar - 140.
Baker BM, Lindsay BD, Bromberg BI, et al. . Catheter ablation of clinical intraatrial reentrant tachycardias resulting from previous atrial surgery: localizing and transecting the critical isthmus. J Am Coll Cardiol . 1996; 28:411–7.CrossrefMedlineGoogle Scholar - 141.
Haines DE, Beheiry S, Akar JG, et al. . Heart Rythm Society expert consensus statement on electrophysiology laboratory standards: process, protocols, equipment, personnel, and safety. Heart Rhythm . 2014; 11:e9–51.CrossrefMedlineGoogle Scholar - 142.
Knight BP, Zivin A, Souza J, et al. . A technique for the rapid diagnosis of atrial tachycardia in the electrophysiology laboratory. J Am Coll Cardiol . 1999; 33:775–81.CrossrefMedlineGoogle Scholar - 143.
Horowitz LN, Kay HR, Kutalek SP, et al. . Risks and complications of clinical cardiac electrophysiologic studies: a prospective analysis of 1,000 consecutive patients. J Am Coll Cardiol . 1987; 9:1261–8.CrossrefMedlineGoogle Scholar - 144.
Asirvatham S, Narayan O . Advanced catheter mapping and navigation system. In:, Huang SW, Wood M , editors. Catheter Ablation of Cardiac Arrhythmias . Philadelphia, PA: Saunders/Elsevier, 2006:135–61.CrossrefGoogle Scholar - 145.
Sporton SC, Earley MJ, Nathan AW, et al. . Electroanatomic versus fluoroscopic mapping for catheter ablation procedures: a prospective randomized study. J Cardiovasc Electrophysiol . 2004; 15:310–5.CrossrefMedlineGoogle Scholar - 146.
Kopelman HA, Prater SP, Tondato F, et al. . Slow pathway catheter ablation of atrioventricular nodal re-entrant tachycardia guided by electroanatomical mapping: a randomized comparison to the conventional approach. Europace . 2003; 5:171–4.CrossrefMedlineGoogle Scholar - 147.
Hindricks G, Willems S, Kautzner J, et al. . Effect of electroanatomically guided versus conventional catheter ablation of typical atrial flutter on the fluoroscopy time and resource use: a prospective randomized multicenter study. J Cardiovasc Electrophysiol . 2009; 20:734–40.CrossrefMedlineGoogle Scholar - 148.
Mah DY, Miyake CY, Sherwin ED, et al. . The use of an integrated electroanatomic mapping system and intracardiac echocardiography to reduce radiation exposure in children and young adults undergoing ablation of supraventricular tachycardia. Europace . 2014; 16:277–83.CrossrefMedlineGoogle Scholar - 149.
Alvarez M, Tercedor L, Almansa I, et al. . Safety and feasibility of catheter ablation for atrioventricular nodal re-entrant tachycardia without fluoroscopic guidance. Heart Rhythm . 2009; 6:1714–20.CrossrefMedlineGoogle Scholar - 150.
Casella M, Pelargonio G, Dello RA, et al. . "Near-zero" fluoroscopic exposure in supraventricular arrhythmia ablation using the EnSite NavX™ mapping system: personal experience and review of the literature. J Interv Card Electrophysiol . 2011; 31:109–18.CrossrefMedlineGoogle Scholar - 151.
Razminia M, Manankil MF, Eryazici PLS, et al. . Nonfluoroscopic catheter ablation of cardiac arrhythmias in adults: feasibility, safety, and efficacy. J Cardiovasc Electrophysiol . 2012; 23:1078–86.CrossrefMedlineGoogle Scholar - 152.
Earley MJ, Showkathali R, Alzetani M, et al. . Radiofrequency ablation of arrhythmias guided by non-fluoroscopic catheter location: a prospective randomized trial. Eur Heart J . 2006; 27:1223–9.CrossrefMedlineGoogle Scholar - 153.
Xu D, Yang B, Shan Q, et al. . Initial clinical experience of remote magnetic navigation system for catheter mapping and ablation of supraventricular tachycardias. J Interv Card Electrophysiol . 2009; 25:171–4.CrossrefMedlineGoogle Scholar - 154.
Sommer P, Wojdyla-Hordynska A, Rolf S, et al. . Initial experience in ablation of typical atrial flutter using a novel three-dimensional catheter tracking system. Europace . 2013; 15:578–81.CrossrefMedlineGoogle Scholar - 155.
Cummings JE, Pacifico A, Drago JL, et al. . Alternative energy sources for the ablation of arrhythmias. Pacing Clin Electrophysiol . 2005; 28:434–43.CrossrefMedlineGoogle Scholar - 156.
Hanninen M, Yeung-Lai-Wah N, Massel D, et al. . Cryoablation versus RF ablation for AVNRT: a meta-analysis and systematic review. J Cardiovasc Electrophysiol . 2013; 24:1354–60.CrossrefMedlineGoogle Scholar - 157.
Friedman PL, Dubuc M, Green MS, et al. . Catheter cryoablation of supraventricular tachycardia: results of the multicenter prospective "frosty" trial. Heart Rhythm . 2004; 1:129–38.CrossrefMedlineGoogle Scholar - 158.
Rodriguez-Entem FJ, Expósito V, Gonzalez-Enriquez S, et al. . Cryoablation versus radiofrequency ablation for the treatment of atrioventricular nodal reentrant tachycardia: results of a prospective randomized study. J Interv Card Electrophysiol . 2013; 36:41–5.CrossrefMedlineGoogle Scholar - 159.
Eckhardt LLL, Leal M, Hollis Z, et al. . Cryoablation for AVNRT: importance of ablation endpoint criteria. J Cardiovasc Electrophysiol . 2012; 23:729–34.CrossrefMedlineGoogle Scholar - 160.
Olshansky B, Sullivan RM . Inappropriate sinus tachycardia. J Am Coll Cardiol . 2013; 61:793–801.CrossrefMedlineGoogle Scholar - 161.
Marcus B, Gillette PC, Garson A . Intrinsic heart rate in children and young adults: an index of sinus node function isolated from autonomic control. Am Heart J . 1990; 119:911–6.CrossrefMedlineGoogle Scholar - 162.
Jose AD, Collison D . The normal range and determinants of the intrinsic heart rate in man. Cardiovasc Res . 1970; 4:160–7.CrossrefMedlineGoogle Scholar - 163.
Alboni P, Malcarne C, Pedroni P, et al. . Electrophysiology of normal sinus node with and without autonomic blockade. Circulation . 1982; 65:1236–42.CrossrefMedlineGoogle Scholar - 164.
Winum P-F, Cayla G, Rubini M, et al. . A case of cardiomyopathy induced by inappropriate sinus tachycardia and cured by ivabradine. Pacing Clin Electrophysiol . 2009; 32:942–4.CrossrefMedlineGoogle Scholar - 165.
Romeo E, Grimaldi N, Sarubbi B, et al. . A pediatric case of cardiomyopathy induced by inappropriate sinus tachycardia: efficacy of ivabradine. Pediatr Cardiol . 2011; 32:842–5.CrossrefMedlineGoogle Scholar - 166.
Fox K, Ford I, Steg PG, et al. . Ivabradine for patients with stable coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a randomised, double-blind, placebo-controlled trial. Lancet . 2008; 372:807–16.CrossrefMedlineGoogle Scholar - 167.
Swedberg K, Komajda M, Böhm M, et al. . Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet . 2010; 376:875–85.CrossrefMedlineGoogle Scholar - 168.
Cappato R, Castelvecchio S, Ricci C, et al. . Clinical efficacy of ivabradine in patients with inappropriate sinus tachycardia: a prospective, randomized, placebo-controlled, double-blind, crossover evaluation. J Am Coll Cardiol . 2012; 60:1323–9.CrossrefMedlineGoogle Scholar - 169.
Benezet-Mazuecos J, Rubio JM, Farré J, et al. . Long-term outcomes of ivabradine in inappropriate sinus tachycardia patients: appropriate efficacy or inappropriate patients. Pacing Clin Electrophysiol . 2013; 36:830–6.CrossrefMedlineGoogle Scholar - 170.
Ptaszynski P, Kaczmarek K, Ruta J, et al. . Metoprolol succinate vs. ivabradine in the treatment of inappropriate sinus tachycardia in patients unresponsive to previous pharmacological therapy. Europace . 2013; 15:116–21.CrossrefMedlineGoogle Scholar - 171.
Ptaszynski P, Kaczmarek K, Ruta J, et al. . Ivabradine in the treatment of inappropriate sinus tachycardia in patients after successful radiofrequency catheter ablation of atrioventricular node slow pathway. Pacing Clin Electrophysiol . 2013; 36:42–9.CrossrefMedlineGoogle Scholar - 172.
Ptaszynski P, Kaczmarek K, Ruta J, et al. . Ivabradine in combination with metoprolol succinate in the treatment of inappropriate sinus tachycardia. J Cardiovasc Pharmacol Ther . 2013; 18:338–44.CrossrefMedlineGoogle Scholar - 173.
Calò L, Rebecchi M, Sette A, et al. . Efficacy of ivabradine administration in patients affected by inappropriate sinus tachycardia. Heart Rhythm . 2010; 7:1318–23.CrossrefMedlineGoogle Scholar - 174.
Kaplinsky E, Comes FP, Urondo LSV, et al. . Efficacy of ivabradine in four patients with inappropriate sinus tachycardia: a three month-long experience based on electrocardiographic, Holter monitoring, exercise tolerance and quality of life assessments. Cardiol J . 2010; 17:166–71.MedlineGoogle Scholar - 175.
Rakovec P . Treatment of inappropriate sinus tachycardia with ivabradine. Wien Klin Wochenschr . 2009; 121:715–8.CrossrefMedlineGoogle Scholar - 176.
Zellerhoff S, Hinterseer M, Felix Krull B, et al. . Ivabradine in patients with inappropriate sinus tachycardia. Naunyn Schmiedebergs Arch Pharmacol . 2010; 382:483–6.CrossrefMedlineGoogle Scholar - 177.
Man KC, Knight B, Tse HF, et al. . Radiofrequency catheter ablation of inappropriate sinus tachycardia guided by activation mapping. J Am Coll Cardiol . 2000; 35:451–7.CrossrefMedlineGoogle Scholar - 178.
Lin D, Garcia F, Jacobson J, et al. . Use of noncontact mapping and saline-cooled ablation catheter for sinus node modification in medically refractory inappropriate sinus tachycardia. Pacing Clin Electrophysiol . 2007; 30:236–42.CrossrefMedlineGoogle Scholar - 179.
Lee RJ, Kalman JM, Fitzpatrick AP, et al. . Radiofrequency catheter modification of the sinus node for "inappropriate" sinus tachycardia. Circulation . 1995; 92:2919–28.CrossrefMedlineGoogle Scholar - 180.
Marrouche NF, Beheiry S, Tomassoni G, et al. . Three-dimensional nonfluoroscopic mapping and ablation of inappropriate sinus tachycardia. Procedural strategies and long-term outcome. J Am Coll Cardiol . 2002; 39:1046–54.CrossrefMedlineGoogle Scholar - 181.
Callans DJ, Ren JF, Schwartzman D, et al. . Narrowing of the superior vena cava-right atrium junction during radiofrequency catheter ablation for inappropriate sinus tachycardia: analysis with intracardiac echocardiography. J Am Coll Cardiol . 1999; 33:1667–70.CrossrefMedlineGoogle Scholar - 182.
Frankel DS, Lin D, Anastasio N, et al. . Frequent additional tachyarrhythmias in patients with inappropriate sinus tachycardia undergoing sinus node modification: an important cause of symptom recurrence. J Cardiovasc Electrophysiol . 2012; 23:835–9.CrossrefMedlineGoogle Scholar - 183.
Takemoto M, Mukai Y, Inoue S, et al. . Usefulness of non-contact mapping for radiofrequency catheter ablation of inappropriate sinus tachycardia: new procedural strategy and long-term clinical outcome. Intern Med . 2012; 51:357–62.CrossrefMedlineGoogle Scholar - 184.
Koplan BA, Parkash R, Couper G, et al. . Combined epicardial-endocardial approach to ablation of inappropriate sinus tachycardia. J Cardiovasc Electrophysiol . 2004; 15:237–40.CrossrefMedlineGoogle Scholar - 185.
Klein I, Ojamaa K . Thyroid hormone and the cardiovascular system. N Engl J Med . 2001; 344:501–9.CrossrefMedlineGoogle Scholar - 186.
Steinbeck G, Hoffmann E . 'True' atrial tachycardia. Eur Heart J . 1998; 19Suppl E:E48–9.Google Scholar - 187.
Wren C . Incessant tachycardias. Eur Heart J . 1998; 19Suppl E:E54–9.Google Scholar - 188.
Medi C, Kalman JM, Haqqani H, et al. . Tachycardia-mediated cardiomyopathy secondary to focal atrial tachycardia: long-term outcome after catheter ablation. J Am Coll Cardiol . 2009; 53:1791–7.CrossrefMedlineGoogle Scholar - 189.
Tang CW, Scheinman MM, Van Hare GF, et al. . Use of P wave configuration during atrial tachycardia to predict site of origin. J Am Coll Cardiol . 1995; 26:1315–24.CrossrefMedlineGoogle Scholar - 190.
Kistler PM, Roberts-Thomson KC, Haqqani HM, et al. . P-wave morphology in focal atrial tachycardia: development of an algorithm to predict the anatomic site of origin. J Am Coll Cardiol . 2006; 48:1010–7.CrossrefMedlineGoogle Scholar - 191.
Liu X, Dong J, Ho SY, et al. . Atrial tachycardia arising adjacent to noncoronary aortic sinus: distinctive atrial activation patterns and anatomic insights. J Am Coll Cardiol . 2010; 56:796–804.CrossrefMedlineGoogle Scholar - 192.
Morton JB, Sanders P, Das A, et al. . Focal atrial tachycardia arising from the tricuspid annulus: electrophysiologic and electrocardiographic characteristics. J Cardiovasc Electrophysiol . 2001; 12:653–9.CrossrefMedlineGoogle Scholar - 193.
Kistler PM, Sanders P, Hussin A, et al. . Focal atrial tachycardia arising from the mitral annulus: electrocardiographic and electrophysiologic characterization. J Am Coll Cardiol . 2003; 41:2212–9.CrossrefMedlineGoogle Scholar - 194.
Gonzalez MD, Contreras LJ, Jongbloed MRM, et al. . Left atrial tachycardia originating from the mitral annulus-aorta junction. Circulation . 2004; 110: 3187–92.LinkGoogle Scholar - 195.
Ouyang F, Ma J, Ho SY, et al. . Focal atrial tachycardia originating from the non-coronary aortic sinus: electrophysiological characteristics and catheter ablation. J Am Coll Cardiol . 2006; 48:122–31.CrossrefMedlineGoogle Scholar - 196.
Roberts-Thomson KC, Kistler PM, Haqqani HM, et al. . Focal atrial tachycardias arising from the right atrial appendage: electrocardiographic and electrophysiologic characteristics and radiofrequency ablation. J Cardiovasc Electrophysiol . 2007; 18:367–72.CrossrefMedlineGoogle Scholar - 197.
Walters TE, Kistler PM, Kalman JM . Radiofrequency ablation for atrial tachycardia and atrial flutter. Heart Lung Circ . 2012; 21:386–94.CrossrefMedlineGoogle Scholar - 198.
Lee G, Sanders P, Kalman JM . Catheter ablation of atrial arrhythmias: state of the art. Lancet . 2012; 380:1509–19.CrossrefMedlineGoogle Scholar - 199.
Markowitz SM, Nemirovksy D, Stein KM, et al. . Adenosine-insensitive focal atrial tachycardia: evidence for de novo micro-re-entry in the human atrium. J Am Coll Cardiol . 2007; 49:1324–33.CrossrefMedlineGoogle Scholar - 200.
Engelstein ED, Lippman N, Stein KM, et al. . Mechanism-specific effects of adenosine on atrial tachycardia. Circulation . 1994; 89:2645–54.CrossrefMedlineGoogle Scholar - 201.
Cossú SF, Steinberg JS . Supraventricular tachyarrhythmias involving the sinus node: clinical and electrophysiologic characteristics. Prog Cardiovasc Dis . 1998; 41:51–63.CrossrefMedlineGoogle Scholar - 202.
Goya M, Iesaka Y, Takahashi A, et al. . Radiofrequency catheter ablation for sinoatrial node reentrant tachycardia: electrophysiologic features of ablation sites. Jpn Circ J . 1999; 63:177–83.CrossrefMedlineGoogle Scholar - 203.
Saoudi N, Cosío F, Waldo A, et al. . A classification of atrial flutter and regular atrial tachycardia according to electrophysiological mechanisms and anatomical bases: a statement from a joint expert group from the Working Group of Arrhythmias of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J . 2001; 22:1162–82.MedlineGoogle Scholar - 204.
Gillette PC, Garson A Electrophysiologic and pharmacologic characteristics of automatic ectopic atrial tachycardia. Circulation . 1977; 56:571–5.LinkGoogle Scholar - 205.
Mehta AV, Sanchez GR, Sacks EJ, et al. . Ectopic automatic atrial tachycardia in children: clinical characteristics, management and follow-up. J Am Coll Cardiol . 1988; 11:379–85.CrossrefMedlineGoogle Scholar - 206.
Eidher U, Freihoff F, Kaltenbrunner W, et al. . Efficacy and safety of ibutilide for the conversion of monomorphic atrial tachycardia. Pacing Clin Electrophysiol . 2006; 29:358–62.CrossrefMedlineGoogle Scholar - 207.
Markowitz SM, Stein KM, Mittal S, et al. . Differential effects of adenosine on focal and macroreentrant atrial tachycardia. J Cardiovasc Electrophysiol . 1999; 10:489–502.CrossrefMedlineGoogle Scholar - 208.
Reisinger J, Gstrein C, Winter T, et al. . Optimization of initial energy for cardioversion of atrial tachyarrhythmias with biphasic shocks. Am J Emerg Med . 2010; 28:159–65.CrossrefMedlineGoogle Scholar - 209.
Kunze KP, Kuck KH, Schlüter M, et al. . Effect of encainide and flecainide on chronic ectopic atrial tachycardia. J Am Coll Cardiol . 1986; 7:1121–6.CrossrefMedlineGoogle Scholar - 210.
Creamer JE, Nathan AW, Camm AJ . Successful treatment of atrial tachycardias with flecainide acetate. Br Heart J . 1985; 53:164–6.CrossrefMedlineGoogle Scholar - 211.
von Bernuth G, Engelhardt W, Kramer HH, et al. . Atrial automatic tachycardia in infancy and childhood. Eur Heart J . 1992; 13:1410–5.CrossrefMedlineGoogle Scholar - 212.
Lucet V, Do Ngoc D, Fidelle J, et al. . [Anti-arrhythmia efficacy of propafenone in children. Apropos of 30 cases]. Arch Mal Coeur Vaiss . 1987; 80:1385–93.MedlineGoogle Scholar - 213.
Heusch A, Kramer HH, Krogmann ON, et al. . Clinical experience with propafenone for cardiac arrhythmias in the young. Eur Heart J . 1994; 15:1050–6.CrossrefMedlineGoogle Scholar - 214.
Janousek J, Paul T . Safety of oral propafenone in the treatment of arrhythmias in infants and children (European retrospective multicenter study). Working Group on Pediatric Arrhythmias and Electrophysiology of the Association of European Pediatric Cardiologists. Am J Cardiol . 1998; 81:1121–4.CrossrefMedlineGoogle Scholar - 215.
Colloridi V, Perri C, Ventriglia F, et al. . Oral sotalol in pediatric atrial ectopic tachycardia. Am Heart J . 1992; 123:254–6.CrossrefMedlineGoogle Scholar - 216.
Guccione P, Paul T, Garson A Long-term follow-up of amiodarone therapy in the young: continued efficacy, unimpaired growth, moderate side effects. J Am Coll Cardiol . 1990; 15:1118–24.CrossrefMedlineGoogle Scholar - 217.
Coumel P, Fidelle J . Amiodarone in the treatment of cardiac arrhythmias in children: one hundred thirty-five cases. Am Heart J . 1980; 100:1063–9.CrossrefMedlineGoogle Scholar - 218.
Miyazaki A, Ohuchi H, Kurosaki K, et al. . Efficacy and safety of sotalol for refractory tachyarrhythmias in congenital heart disease. Circ J . 2008; 72:1998–2003.CrossrefMedlineGoogle Scholar - 219.
Kang KT, Etheridge SP, Kantoch MJ, et al. . Current management of focal atrial tachycardia in children: a multicenter experience. Circ Arrhythm Electrophysiol . 2014; 7:664–70.LinkGoogle Scholar - 220.
Kothari SA, Apiyasawat S, Asad N, et al. . Evidence supporting a new rate threshold for multifocal atrial tachycardia. Clin Cardiol . 2005; 28:561–3.CrossrefMedlineGoogle Scholar - 221.
Yokoshiki H, Mitsuyama H, Watanabe M, et al. . Swallowing-induced multifocal atrial tachycardia originating from right pulmonary veins. J Electrocardiol . 2011; 44:395.e1–5.CrossrefMedlineGoogle Scholar - 222.
Marchlinski FE, Miller JM . Atrial arrhythmias exacerbated by theophylline. Response to verapamil and evidence for triggered activity in man. Chest . 1985; 88:931–4.CrossrefMedlineGoogle Scholar - 223.
Wang K, Goldfarb BL, Gobel FL, et al. . Multifocal atrial tachycardia. Arch Intern Med . 1977; 137:161–4.CrossrefMedlineGoogle Scholar - 224.
Bittar G, Friedman HS . The arrhythmogenicity of theophylline. A multivariate analysis of clinical determinants. Chest . 1991; 99:1415–20.CrossrefMedlineGoogle Scholar - 225.
Iseri LT, Fairshter RD, Hardemann JL, et al. . Magnesium and potassium therapy in multifocal atrial tachycardia. Am Heart J . 1985; 110:789–94.CrossrefMedlineGoogle Scholar - 226.
Kastor JA . Multifocal atrial tachycardia. N Engl J Med . 1990; 322:1713–7.CrossrefMedlineGoogle Scholar - 227.
Hazard PB, Burnett CR . Verapamil in multifocal atrial tachycardia. Hemodynamic and respiratory changes. Chest . 1987; 91:68–70.CrossrefMedlineGoogle Scholar - 228.
Mukerji V, Alpert MA, Diaz-Arias M, et al. . Termination and suppression of multifocal atrial tachycardia with verapamil. South Med J . 1987; 80:269–70.CrossrefMedlineGoogle Scholar - 229.
Arsura EL, Solar M, Lefkin AS, et al. . Metoprolol in the treatment of multifocal atrial tachycardia. Crit Care Med . 1987; 15:591–4.CrossrefMedlineGoogle Scholar - 230.
Hazard PB, Burnett CR . Treatment of multifocal atrial tachycardia with metoprolol. Crit Care Med . 1987; 15:20–5.CrossrefMedlineGoogle Scholar - 231.
Kouvaras G, Cokkinos DV, Halal G, et al. . The effective treatment of multifocal atrial tachycardia with amiodarone. Jpn Heart J . 1989; 30:301–12.CrossrefMedlineGoogle Scholar - 232.
Levine JH, Michael JR, Guarnieri T . Treatment of multifocal atrial tachycardia with verapamil. N Engl J Med . 1985; 312:21–5.CrossrefMedlineGoogle Scholar - 233.
Salerno DM, Anderson B, Sharkey PJ, et al. . Intravenous verapamil for treatment of multifocal atrial tachycardia with and without calcium pretreatment. Ann Intern Med . 1987; 107:623–8.CrossrefMedlineGoogle Scholar - 234.
Mehta D, Wafa S, Ward DE, et al. . Relative efficacy of various physical manoeuvres in the termination of junctional tachycardia. Lancet . 1988; 1:1181–5.CrossrefMedlineGoogle Scholar - 235.
Wen ZC, Chen SA, Tai CT, et al. . Electrophysiological mechanisms and determinants of vagal maneuvers for termination of paroxysmal supraventricular tachycardia. Circulation . 1998; 98:2716–23.CrossrefMedlineGoogle Scholar - 236.
Glatter KA, Cheng J, Dorostkar P, et al. . Electrophysiologic effects of adenosine in patients with supraventricular tachycardia. Circulation . 1999; 99:1034–40.CrossrefMedlineGoogle Scholar - 237.
Dougherty AH, Jackman WM, Naccarelli GV, et al. . Acute conversion of paroxysmal supraventricular tachycardia with intravenous diltiazem. IV Diltiazem Study Group. Am J Cardiol . 1992; 70:587–92.CrossrefMedlineGoogle Scholar - 238.
Waxman HL, Myerburg RJ, Appel R, et al. . Verapamil for control of ventricular rate in paroxysmal supraventricular tachycardia and atrial fibrillation or flutter: a double-blind randomized cross-over study. Ann Intern Med . 1981; 94:1–6.CrossrefMedlineGoogle Scholar - 239.
Amsterdam EA, Kulcyski J, Ridgeway MG . Efficacy of cardioselective beta-adrenergic blockade with intravenously administered metoprolol in the treatment of supraventricular tachyarrhythmias. J Clin Pharmacol . 1991; 31:714–8.CrossrefMedlineGoogle Scholar - 240.
Das G, Tschida V, Gray R, et al. . Efficacy of esmolol in the treatment and transfer of patients with supraventricular tachyarrhythmias to alternate oral antiarrhythmic agents. J Clin Pharmacol . 1988; 28:746–50.CrossrefMedlineGoogle Scholar - 241.
Alboni P, Tomasi C, Menozzi C, et al. . Efficacy and safety of out-of-hospital self-administered single-dose oral drug treatment in the management of infrequent, well-tolerated paroxysmal supraventricular tachycardia. J Am Coll Cardiol . 2001; 37:548–53.CrossrefMedlineGoogle Scholar - 242.
Yeh SJ, Lin FC, Chou YY, et al. . Termination of paroxysmal supraventricular tachycardia with a single oral dose of diltiazem and propranolol. Circulation . 1985; 71:104–9.CrossrefMedlineGoogle Scholar - 243.
Rinkenberger RL, Prystowsky EN, Heger JJ, et al. . Effects of intravenous and chronic oral verapamil administration in patients with supraventricular tachyarrhythmias. Circulation . 1980; 62:996–1010.CrossrefMedlineGoogle Scholar - 244.
D'Este D, Zoppo F, Bertaglia E, et al. . Long-term outcome of patients with atrioventricular node reentrant tachycardia. Int J Cardiol . 2007; 115:350–3.CrossrefMedlineGoogle Scholar - 245.
Langberg JJ, Leon A, Borganelli M, et al. . A randomized, prospective comparison of anterior and posterior approaches to radiofrequency catheter ablation of atrioventricular nodal reentry tachycardia. Circulation . 1993; 87:1551–6.CrossrefMedlineGoogle Scholar - 246.
Kalbfleisch SJ, Strickberger SA, Williamson B, et al. . Randomized comparison of anatomic and electrogram mapping approaches to ablation of the slow pathway of atrioventricular node reentrant tachycardia. J Am Coll Cardiol . 1994; 23:716–23.CrossrefMedlineGoogle Scholar - 247.
Kay GN, Epstein AE, Dailey SM, et al. . Selective radiofrequency ablation of the slow pathway for the treatment of atrioventricular nodal reentrant tachycardia. Evidence for involvement of perinodal myocardium within the reentrant circuit. Circulation . 1992; 85:1675–88.CrossrefMedlineGoogle Scholar - 248.
Bogun F, Knight B, Weiss R, et al. . Slow pathway ablation in patients with documented but noninducible paroxysmal supraventricular tachycardia. J Am Coll Cardiol . 1996; 28:1000–4.CrossrefMedlineGoogle Scholar - 249.
O'Hara GE, Philippon F, Champagne J, et al. . Catheter ablation for cardiac arrhythmias: a 14-year experience with 5330 consecutive patients at the Quebec Heart Institute, Laval Hospital. Can J Cardiol . 2007; 23Suppl B:67B–70B.CrossrefMedlineGoogle Scholar - 250.
Gambhir DS, Bhargava M, Arora R, et al. . Electrophysiologic effects and therapeutic efficacy of intravenous flecainide for termination of paroxysmal supraventricular tachycardia. Indian Heart J . 1995; 47:237–43.MedlineGoogle Scholar - 251.
Musto B, Cavallaro C, Musto A, et al. . Flecainide single oral dose for management of paroxysmal supraventricular tachycardia in children and young adults. Am Heart J . 1992; 124:110–5.CrossrefMedlineGoogle Scholar - 252.
Wellens HJ . Should catheter ablation be performed in asymptomatic patients with Wolff-Parkinson-White syndrome? When to perform catheter ablation in asymptomatic patients with a Wolff-Parkinson-White electrocardiogram. Circulation . 2005; 112:2201–7.LinkGoogle Scholar - 253.
Munger TM, Packer DL, Hammill SC, et al. . A population study of the natural history of Wolff-Parkinson-White syndrome in Olmsted County, Minnesota, 1953–1989. Circulation . 1993; 87:866–73.CrossrefMedlineGoogle Scholar - 254.
Pappone C, Vicedomini G, Manguso F, et al. . Wolff-Parkinson-White syndrome in the era of catheter ablation: insights from a registry study of 2169 patients. Circulation . 2014; 130:811–9.LinkGoogle Scholar - 255.
Timmermans C, Smeets JL, Rodriguez LM, et al. . Aborted sudden death in the Wolff-Parkinson-White syndrome. Am J Cardiol . 1995; 76:492–4.CrossrefMedlineGoogle Scholar - 256.
Klein GJ, Bashore TM, Sellers TD, et al. . Ventricular fibrillation in the Wolff-Parkinson-White syndrome. N Engl J Med . 1979; 301:1080–5.CrossrefMedlineGoogle Scholar - 257.
Bromberg BI, Lindsay BD, Cain ME, et al. . Impact of clinical history and electrophysiologic characterization of accessory pathways on management strategies to reduce sudden death among children with Wolff-Parkinson-White syndrome. J Am Coll Cardiol . 1996; 27:690–5.CrossrefMedlineGoogle Scholar - 258.
Santinelli V, Radinovic A, Manguso F, et al. . The natural history of asymptomatic ventricular pre-excitation: a long-term prospective follow-up study of 184 asymptomatic children. J Am Coll Cardiol . 2009; 53:275–80.CrossrefMedlineGoogle Scholar - 259.
Smith GD, Dyson K, Taylor D, et al. . Effectiveness of the Valsalva manoeuvre for reversion of supraventricular tachycardia. Cochrane Database Syst Rev . 2013; 3:CD009502.Google Scholar - 260.
Delaney B, Loy J, Kelly A-M . The relative efficacy of adenosine versus verapamil for the treatment of stable paroxysmal supraventricular tachycardia in adults: a meta-analysis. Eur J Emerg Med . 2011; 18:148–52.CrossrefMedlineGoogle Scholar - 261.
Furlong R, Gerhardt RT, Farber P, et al. . Intravenous adenosine as first-line prehospital management of narrow-complex tachycardias by EMS personnel without direct physician control. Am J Emerg Med . 1995; 13:383–8.CrossrefMedlineGoogle Scholar - 262.
Schatz I, Ordog GJ, Karody R, et al. . Wolff-Parkinson-White syndrome presenting in atrial fibrillation. Ann Emerg Med . 1987; 16:574–8.CrossrefMedlineGoogle Scholar - 263.
Mittal S, Ayati S, Stein KM, et al. . Transthoracic cardioversion of atrial fibrillation: comparison of rectilinear biphasic versus damped sine wave monophasic shocks. Circulation . 2000; 101:1282–7.CrossrefMedlineGoogle Scholar - 264.
Glatter KA, Dorostkar PC, Yang Y, et al. . Electrophysiological effects of ibutilide in patients with accessory pathways. Circulation . 2001; 104:1933–9.LinkGoogle Scholar - 265.
Sellers TD, Campbell RW, Bashore TM, et al. . Effects of procainamide and quinidine sulfate in the Wolff-Parkinson-White syndrome. Circulation . 1977; 55:15–22.LinkGoogle Scholar - 266.
Huycke EC, Sung RJ, Dias VC, et al. . Intravenous diltiazem for termination of reentrant supraventricular tachycardia: a placebo-controlled, randomized, double-blind, multicenter study. J Am Coll Cardiol . 1989; 13:538–44.CrossrefMedlineGoogle Scholar - 267.
Hamer A, Peter T, Platt M, et al. . Effects of verapamil on supraventricular tachycardia in patients with overt and concealed Wolff-Parkinson-White syndrome. Am Heart J . 1981; 101:600–12.CrossrefMedlineGoogle Scholar - 268.
Hombach V, Braun V, Höpp HW, et al. . Antiarrhythmic effects of acute beta blockade with atenolol on supraventricular tachycardias at rest and during exercise. Klin Wochenschr . 1981; 59:123–33.CrossrefMedlineGoogle Scholar - 269.
Morady F, DiCarlo LA, Baerman JM, et al. . Effect of propranolol on ventricular rate during atrial fibrillation in the Wolff-Parkinson-White syndrome. Pacing Clin Electrophysiol . 1987; 10:492–6.CrossrefMedlineGoogle Scholar - 270.
Wellens HJ, Durrer D . Effect of digitalis on atrioventricular conduction and circus-movement tachycardias in patients with Wolff-Parkinson-White syndrome. Circulation . 1973; 47:1229–33.CrossrefMedlineGoogle Scholar - 271.
Sellers TD, Bashore TM, Gallagher JJ . Digitalis in the pre-excitation syndrome. Analysis during atrial fibrillation. Circulation . 1977; 56:260–7.LinkGoogle Scholar - 272.
Sheinman BD, Evans T . Acceleration of ventricular rate by fibrillation associated with the Wolff-Parkinson-White syndrome. Br Med J (Clin Res Ed) . 1982; 285:999–1000.CrossrefMedlineGoogle Scholar - 273.
Boriani G, Biffi M, Frabetti L, et al. . Ventricular fibrillation after intravenous amiodarone in Wolff-Parkinson-White syndrome with atrial fibrillation. Am Heart J . 1996; 131:1214–6.CrossrefMedlineGoogle Scholar - 274.
Shiraishi H, Ishibashi K, Urao N, et al. . Two cases of polymorphic ventricular tachycardia induced by the administration of verapamil against paroxysmal supraventricular tachycardia. Intern Med . 2002; 41:445–8.CrossrefMedlineGoogle Scholar - 275.
Schützenberger W, Leisch F, Gmeiner R . Enhanced accessory pathway conduction following intravenous amiodarone in atrial fibrillation. A case report. Int J Cardiol . 1987; 16:93–5.CrossrefMedlineGoogle Scholar - 276.
Jackman WM, Wang XZ, Friday KJ, et al. . Catheter ablation of accessory atrioventricular pathways (Wolff-Parkinson-White syndrome) by radiofrequency current. N Engl J Med . 1991; 324:1605–11.CrossrefMedlineGoogle Scholar - 277.
Calkins H, Langberg J, Sousa J, et al. . Radiofrequency catheter ablation of accessory atrioventricular connections in 250 patients. Abbreviated therapeutic approach to Wolff-Parkinson-White syndrome. Circulation . 1992; 85:1337–46.CrossrefMedlineGoogle Scholar - 278.
Dagres N, Clague JR, Kottkamp H, et al. . Radiofrequency catheter ablation of accessory pathways. Outcome and use of antiarrhythmic drugs during follow-up. Eur Heart J . 1999; 20:1826–32.CrossrefMedlineGoogle Scholar - 279.
Schläpfer J, Fromer M . Late clinical outcome after successful radiofrequency catheter ablation of accessory pathways. Eur Heart J . 2001; 22:605–9.CrossrefMedlineGoogle Scholar - 280.
Belhassen B, Rogowski O, Glick A, et al. . Radiofrequency ablation of accessory pathways: a 14 year experience at the Tel Aviv Medical Center in 508 patients. Isr Med Assoc J . 2007; 9:265–70.MedlineGoogle Scholar - 281.
Kugler JD, Danford DA, Deal BJ, et al. . Radiofrequency catheter ablation for tachyarrhythmias in children and adolescents. The Pediatric Electrophysiology Society. N Engl J Med . 1994; 330:1481–7.CrossrefMedlineGoogle Scholar - 282.
Kugler JD, Danford DA, Houston K, et al. . Radiofrequency catheter ablation for paroxysmal supraventricular tachycardia in children and adolescents without structural heart disease. Pediatric EP Society, Radiofrequency Catheter Ablation Registry. Am J Cardiol . 1997; 80:1438–43.CrossrefMedlineGoogle Scholar - 283.
Aguinaga L, Primo J, Anguera I, et al. . Long-term follow-up in patients with the permanent form of junctional reciprocating tachycardia treated with radiofrequency ablation. Pacing Clin Electrophysiol . 1998; 21:2073–8.CrossrefMedlineGoogle Scholar - 284.
Kang KT, Potts JE, Radbill AE, et al. . Permanent junctional reciprocating tachycardia in children: a multicenter experience. Heart Rhythm . 2014; 11:1426–32.CrossrefMedlineGoogle Scholar - 285.
McClelland JH, Wang X, Beckman KJ, et al. . Radiofrequency catheter ablation of right atriofascicular (Mahaim) accessory pathways guided by accessory pathway activation potentials. Circulation . 1994; 89:2655–66.CrossrefMedlineGoogle Scholar - 286.
Mönnig G, Wasmer K, Milberg P, et al. . Predictors of long-term success after catheter ablation of atriofascicular accessory pathways. Heart Rhythm . 2012; 9:704–8.CrossrefMedlineGoogle Scholar - 287.
Sakurai M, Yasuda H, Kato N, et al. . Acute and chronic effects of verapamil in patients with paroxysmal supraventricular tachycardia. Am Heart J . 1983; 105:619–28.CrossrefMedlineGoogle Scholar - 288.
Hopson JR, Buxton AE, Rinkenberger RL, et al. . Safety and utility of flecainide acetate in the routine care of patients with supraventricular tachyarrhythmias: results of a multicenter trial. The Flecainide Supraventricular Tachycardia Study Group. Am J Cardiol . 1996; 77:72A–82A.CrossrefMedlineGoogle Scholar - 289.
Feld GK, Nademanee K, Weiss J, et al. . Electrophysiologic basis for the suppression by amiodarone of orthodromic supraventricular tachycardias complicating pre-excitation syndromes. J Am Coll Cardiol . 1984; 3:1298–307.CrossrefMedlineGoogle Scholar - 290.
Feld GK, Nademanee K, Stevenson W, et al. . Clinical and electrophysiologic effects of amiodarone in patients with atrial fibrillation complicating the Wolff-Parkinson-White syndrome. Am Heart J . 1988; 115:102–7.CrossrefMedlineGoogle Scholar - 291.
Gemma LW, Steinberg LA, Prystowsky EN, et al. . Development of rapid preexcited ventricular response to atrial fibrillation in a patient with intermittent preexcitation. J Cardiovasc Electrophysiol . 2013; 24:347–50.CrossrefMedlineGoogle Scholar - 292.
Bauernfeind RA, Wyndham CR, Dhingra RC, et al. . Serial electrophysiologic testing of multiple drugs in patients with atrioventricular nodal reentrant paroxysmal tachycardia. Circulation . 1980; 62:1341–9.CrossrefMedlineGoogle Scholar - 293.
Hung JS, Kou HC, Wu D . Digoxin, propranolol, and atrioventricular reentrant tachycardia in the Wolff-Parkinson-White syndrome. Ann Intern Med . 1982; 97:175–82.CrossrefMedlineGoogle Scholar - 294.
Sharma AD, Yee R, Guiraudon G, et al. . Sensitivity and specificity of invasive and noninvasive testing for risk of sudden death in Wolff-Parkinson-White syndrome. J Am Coll Cardiol . 1987; 10:373–81.CrossrefMedlineGoogle Scholar - 295.
Gaita F, Giustetto C, Riccardi R, et al. . Stress and pharmacologic tests as methods to identify patients with Wolff-Parkinson-White syndrome at risk of sudden death. Am J Cardiol . 1989; 64:487–90.CrossrefMedlineGoogle Scholar - 296.
Spar DS, Silver ES, Hordof AJ, et al. . Relation of the utility of exercise testing for risk assessment in pediatric patients with ventricular preexcitation to pathway location. Am J Cardiol . 2012; 109:1011–4.CrossrefMedlineGoogle Scholar - 297.
Wackel P, Irving C, Webber S, et al. . Risk stratification in Wolff-Parkinson-White syndrome: the correlation between noninvasive and invasive testing in pediatric patients. Pacing Clin Electrophysiol . 2012; 35:1451–7.CrossrefMedlineGoogle Scholar - 298.
Beckman KJ, Gallastegui JL, Bauman JL, et al. . The predictive value of electrophysiologic studies in untreated patients with Wolff-Parkinson-White syndrome. J Am Coll Cardiol . 1990; 15:640–7.CrossrefMedlineGoogle Scholar - 299.
Pappone C, Vicedomini G, Manguso F, et al. . Risk of malignant arrhythmias in initially symptomatic patients with Wolff-Parkinson-White syndrome: results of a prospective long-term electrophysiological follow-up study. Circulation . 2012; 125:661–8.LinkGoogle Scholar - 300.
Rinne C, Klein GJ, Sharma AD, et al. . Relation between clinical presentation and induced arrhythmias in the Wolff-Parkinson-White syndrome. Am J Cardiol . 1987; 60:576–9.CrossrefMedlineGoogle Scholar - 301.
Brembilla-Perrot B, Holban I, Houriez P, et al. . Influence of age on the potential risk of sudden death in asymptomatic Wolff-Parkinson-White syndrome. Pacing Clin Electrophysiol . 2001; 24:1514–8.CrossrefMedlineGoogle Scholar - 302.
Pappone C, Santinelli V, Manguso F, et al. . A randomized study of prophylactic catheter ablation in asymptomatic patients with the Wolff-Parkinson-White syndrome. N Engl J Med . 2003; 349:1803–11.CrossrefMedlineGoogle Scholar - 303.
Pappone C, Santinelli V, Rosanio S, et al. . Usefulness of invasive electrophysiologic testing to stratify the risk of arrhythmic events in asymptomatic patients with Wolff-Parkinson-White pattern: results from a large prospective long-term follow-up study. J Am Coll Cardiol . 2003; 41:239–44.CrossrefMedlineGoogle Scholar - 304.
Epstein AE, Miles WM, Benditt DG, et al. . Personal and public safety issues related to arrhythmias that may affect consciousness: implications for regulation and physician recommendations. A medical/scientific statement from the American Heart Association and the North American Society of Pacing and Electrophysiology. Circulation . 1996; 94:1147–66.CrossrefMedlineGoogle Scholar - 305.
Pelliccia A, Zipes DP, Maron BJ . Bethesda Conference #36 and the European Society of Cardiology Consensus Recommendations revisited a comparison of US and European criteria for eligibility and disqualification of competitive athletes with cardiovascular abnormalities. J Am Coll Cardiol . 2008; 52:1990–6.CrossrefMedlineGoogle Scholar - 306.
Klein GJ, Yee R, Sharma AD . Longitudinal electrophysiologic assessment of asymptomatic patients with the Wolff-Parkinson-White electrocardiographic pattern. N Engl J Med . 1989; 320:1229–33.CrossrefMedlineGoogle Scholar - 307.
Leitch JW, Klein GJ, Yee R, et al. . Prognostic value of electrophysiology testing in asymptomatic patients with Wolff-Parkinson-White pattern. Circulation . 1990; 82:1718–23.CrossrefMedlineGoogle Scholar - 308.
Milstein S, Sharma AD, Klein GJ . Electrophysiologic profile of asymptomatic Wolff-Parkinson-White pattern. Am J Cardiol . 1986; 57:1097–100.CrossrefMedlineGoogle Scholar - 309.
Satoh M, Aizawa Y, Funazaki T, et al. . Electrophysiologic evaluation of asymptomatic patients with the Wolff-Parkinson-White pattern. Pacing Clin Electrophysiol . 1989; 12:413–20.CrossrefMedlineGoogle Scholar - 310.
Havránek S, Simek J, St'ovíček P, et al. . Distribution of mean cycle length in cavo-tricuspid isthmus dependent atrial flutter. Physiol Res . 2012; 61:43–51.MedlineGoogle Scholar - 311.
Waldo AL, Feld GK . Inter-relationships of atrial fibrillation and atrial flutter mechanisms and clinical implications. J Am Coll Cardiol . 2008; 51:779–86.CrossrefMedlineGoogle Scholar - 312.
Ellis K, Wazni O, Marrouche N, et al. . Incidence of atrial fibrillation post-cavotricuspid isthmus ablation in patients with typical atrial flutter: left-atrial size as an independent predictor of atrial fibrillation recurrence. J Cardiovasc Electrophysiol . 2007; 18:799–802.CrossrefMedlineGoogle Scholar - 313.
Hsieh M-H, Tai C-T, Chiang C-E, et al. . Recurrent atrial flutter and atrial fibrillation after catheter ablation of the cavotricuspid isthmus: a very long-term follow-up of 333 patients. J Interv Card Electrophysiol . 2002; 7:225–31.CrossrefMedlineGoogle Scholar - 314.
Tai CT, Chen SA, Chiang CE, et al. . Long-term outcome of radiofrequency catheter ablation for typical atrial flutter: risk prediction of recurrent arrhythmias. J Cardiovasc Electrophysiol . 1998; 9:115–21.CrossrefMedlineGoogle Scholar - 315.
Paydak H, Kall JG, Burke MC, et al. . Atrial fibrillation after radiofrequency ablation of type I atrial flutter: time to onset, determinants, and clinical course. Circulation . 1998; 98:315–22.CrossrefMedlineGoogle Scholar - 316.
Chinitz JS, Gerstenfeld EP, Marchlinski FE, et al. . Atrial fibrillation is common after ablation of isolated atrial flutter during long-term follow-up. Heart Rhythm . 2007; 4:1029–33.CrossrefMedlineGoogle Scholar - 317.
Reithmann C, Hoffmann E, Spitzlberger G, et al. . Catheter ablation of atrial flutter due to amiodarone therapy for paroxysmal atrial fibrillation. Eur Heart J . 2000; 21:565–72.CrossrefMedlineGoogle Scholar - 318.
Bertaglia E, Bonso A, Zoppo F, et al. . Different clinical courses and predictors of atrial fibrillation occurrence after transisthmic ablation in patients with preablation lone atrial flutter, coexistent atrial fibrillation, and drug induced atrial flutter. Pacing Clin Electrophysiol . 2004; 27:1507–12.CrossrefMedlineGoogle Scholar - 319.
Satomi K, Chun KRJ, Tilz R, et al. . Catheter ablation of multiple unstable macroreentrant tachycardia within the right atrium free wall in patients without previous cardiac surgery. Circ Arrhythm Electrophysiol . 2010; 3:24–31.LinkGoogle Scholar - 320.
Seiler J, Schmid DK, Irtel TA, et al. . Dual-loop circuits in postoperative atrial macro re-entrant tachycardias. Heart . 2007; 93:325–30.CrossrefMedlineGoogle Scholar - 321.
Roberts-Thomson KC, Kistler PM, Kalman JM . Focal atrial tachycardia I: clinical features, diagnosis, mechanisms, and anatomic location. Pacing Clin Electrophysiol . 2006; 29:643–52.CrossrefMedlineGoogle Scholar - 322.
Barbato G, Carinci V, Tomasi C, et al. . Is electrocardiography a reliable tool for identifying patients with isthmus-dependent atrial flutter? Europace . 2009; 11:1071–6.CrossrefMedlineGoogle Scholar - 323.
Akar JG, Al-Chekakie MO, Hai A, et al. . Surface electrocardiographic patterns and electrophysiologic characteristics of atrial flutter following modified radiofrequency MAZE procedures. J Cardiovasc Electrophysiol . 2007; 18:349–55.CrossrefMedlineGoogle Scholar - 324.
Chugh A, Latchamsetty R, Oral H, et al. . Characteristics of cavotricuspid isthmus-dependent atrial flutter after left atrial ablation of atrial fibrillation. Circulation . 2006; 113:609–15.LinkGoogle Scholar - 325.
Shah D . ECG manifestations of left atrial flutter. Curr Opin Cardiol . 2009; 24:35–41.CrossrefMedlineGoogle Scholar - 326.
Nakagawa H, Jackman WM . Use of a 3-dimensional electroanatomical mapping system for catheter ablation of macroreentrant right atrial tachycardia following atriotomy. J Electrocardiol . 1999; 32Suppl:16–21.CrossrefMedlineGoogle Scholar - 327.
Delacretaz E, Ganz LI, Soejima K, et al. . Multi atrial maco-re-entry circuits in adults with repaired congenital heart disease: entrainment mapping combined with three-dimensional electroanatomic mapping. J Am Coll Cardiol . 2001; 37:1665–76.CrossrefMedlineGoogle Scholar - 328.
Magnin-Poull I, De Chillou C, Miljoen H, et al. . Mechanisms of right atrial tachycardia occurring late after surgical closure of atrial septal defects. J Cardiovasc Electrophysiol . 2005; 16:681–7.CrossrefMedlineGoogle Scholar - 329.
Kanagasundram AN, Baduashvili A, Liu CF, et al. . A novel criterion for conduction block after catheter ablation of right atrial tachycardia after mitral valve surgery. Circ Arrhythm Electrophysiol . 2013; 6:39–47.LinkGoogle Scholar - 330.
Teh AW, Medi C, Lee G, et al. . Long-term outcome following ablation of atrial flutter occurring late after atrial septal defect repair. Pacing Clin Electrophysiol . 2011; 34:431–5.CrossrefMedlineGoogle Scholar - 331.
McElderry HT, McGiffin DC, Plumb VJ, et al. . Proarrhythmic aspects of atrial fibrillation surgery: mechanisms of postoperative macroreentrant tachycardias. Circulation . 2008; 117:155–62.LinkGoogle Scholar - 332.
Wazni OM, Saliba W, Fahmy T, et al. . Atrial arrhythmias after surgical maze: findings during catheter ablation. J Am Coll Cardiol . 2006; 48:1405–9.CrossrefMedlineGoogle Scholar - 333.
Gerstenfeld EP, Callans DJ, Dixit S, et al. . Mechanisms of organized left atrial tachycardias occurring after pulmonary vein isolation. Circulation . 2004; 110:1351–7.LinkGoogle Scholar - 334.
Chugh A, Oral H, Lemola K, et al. . Prevalence, mechanisms, and clinical significance of macroreentrant atrial tachycardia during and following left atrial ablation for atrial fibrillation. Heart Rhythm . 2005; 2:464–71.CrossrefMedlineGoogle Scholar - 335.
Veenhuyzen GD, Knecht S, O'Neill MD, et al. . Atrial tachycardias encountered during and after catheter ablation for atrial fibrillation: part I: classification, incidence, management. Pacing Clin Electrophysiol . 2009; 32:393–8.CrossrefMedlineGoogle Scholar - 336.
Karch MR, Zrenner B, Deisenhofer I, et al. . Freedom from atrial tachyarrhythmias after catheter ablation of atrial fibrillation: a randomized comparison between 2 current ablation strategies. Circulation . 2005; 111:2875–80.LinkGoogle Scholar - 337.
Sawhney N, Anousheh R, Chen W, et al. . Circumferential pulmonary vein ablation with additional linear ablation results in an increased incidence of left atrial flutter compared with segmental pulmonary vein isolation as an initial approach to ablation of paroxysmal atrial fibrillation. Circ Arrhythm Electrophysiol . 2010; 3:243–8.LinkGoogle Scholar - 338.
Neumann T, Vogt J, Schumacher B, et al. . Circumferential pulmonary vein isolation with the cryoballoon technique results from a prospective 3-center study. J Am Coll Cardiol . 2008; 52:273–8.CrossrefMedlineGoogle Scholar - 339.
Calkins H, Kuck KH, Cappato R, et al. . 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS). Heart Rhythm . 2012; 9:632–96.e21.CrossrefMedlineGoogle Scholar - 340.
Ellenbogen KA, Stambler BS, Wood MA, et al. . Efficacy of intravenous ibutilide for rapid termination of atrial fibrillation and atrial flutter: a dose-response study. J Am Coll Cardiol . 1996; 28:130–6.CrossrefMedlineGoogle Scholar - 341.
Suttorp MJ, Kingma JH, Jessurun ER, et al. . The value of class IC antiarrhythmic drugs for acute conversion of paroxysmal atrial fibrillation or flutter to sinus rhythm. J Am Coll Cardiol . 1990; 16:1722–7.CrossrefMedlineGoogle Scholar - 342.
Stambler BS, Wood MA, Ellenbogen KA, et al. . Efficacy and safety of repeated intravenous doses of ibutilide for rapid conversion of atrial flutter or fibrillation. Ibutilide Repeat Dose Study Investigators. Circulation . 1996; 94:1613–21.LinkGoogle Scholar - 343.
Kingma JH, Suttorp MJ . Acute pharmacologic conversion of atrial fibrillation and flutter: the role of flecainide, propafenone, and verapamil. Am J Cardiol . 1992; 70:56A–60A.CrossrefMedlineGoogle Scholar - 344.
Vos MA, Golitsyn SR, Stangl K, et al. . Superiority of ibutilide (a new class III agent) over DL-sotalol in converting atrial flutter and atrial fibrillation. The Ibutilide/Sotalol Comparator Study Group. Heart . 1998; 79:568–75.CrossrefMedlineGoogle Scholar - 345.
Volgman AS, Carberry PA, Stambler B, et al. . Conversion efficacy and safety of intravenous ibutilide compared with intravenous procainamide in patients with atrial flutter or fibrillation. J Am Coll Cardiol . 1998; 31:1414–9.CrossrefMedlineGoogle Scholar - 346.
Singh S, Zoble RG, Yellen L, et al. . Efficacy and safety of oral dofetilide in converting to and maintaining sinus rhythm in patients with chronic atrial fibrillation or atrial flutter: the Symptomatic Atrial Fibrillation Investigative Research on Dofetilide (SAFIRE-D) study. Circulation . 2000; 102:2385–90.CrossrefMedlineGoogle Scholar - 347.
Blackshear JL, Stambler BS, Strauss WE, et al. . Control of heart rate during transition from intravenous to oral diltiazem in atrial fibrillation or flutter. Am J Cardiol . 1996; 78:1246–50.CrossrefMedlineGoogle Scholar - 348.
Singh BN, Hecht HS, Nademanee K, et al. . Electrophysiologic and hemodynamic effects of slow-channel blocking drugs. Prog Cardiovasc Dis . 1982; 25:103–32.CrossrefMedlineGoogle Scholar - 349.
Akhtar M, Tchou P, Jazayeri M . Use of calcium channel entry blockers in the treatment of cardiac arrhythmias. Circulation . 1989; 80:IV31–9.MedlineGoogle Scholar - 350.
Delle Karth G, Geppert A, Neunteufl T, et al. . Amiodarone versus diltiazem for rate control in critically ill patients with atrial tachyarrhythmias. Crit Care Med . 2001; 29:1149–53.CrossrefMedlineGoogle Scholar - 351.
Ellenbogen KA, Dias VC, Plumb VJ, et al. . A placebo-controlled trial of continuous intravenous diltiazem infusion for 24-hour heart rate control during atrial fibrillation and atrial flutter: a multicenter study. J Am Coll Cardiol . 1991; 18:891–7.CrossrefMedlineGoogle Scholar - 352.
Ellenbogen KA, Dias VC, Cardello FP, et al. . Safety and efficacy of intravenous diltiazem in atrial fibrillation or atrial flutter. Am J Cardiol . 1995; 75:45–9.CrossrefMedlineGoogle Scholar - 353.
Platia EV, Michelson EL, Porterfield JK, et al. . Esmolol versus verapamil in the acute treatment of atrial fibrillation or atrial flutter. Am J Cardiol . 1989; 63:925–9.CrossrefMedlineGoogle Scholar - 354.
Salerno DM, Dias VC, Kleiger RE, et al. . Efficacy and safety of intravenous diltiazem for treatment of atrial fibrillation and atrial flutter. The Diltiazem-Atrial Fibrillation/Flutter Study Group. Am J Cardiol . 1989; 63:1046–51.CrossrefMedlineGoogle Scholar - 355.
Basta M, Klein GJ, Yee R, et al. . Current role of pharmacologic therapy for patients with paroxysmal supraventricular tachycardia. Cardiol Clin . 1997; 15:587–97.CrossrefMedlineGoogle Scholar - 356.
Gallagher MM, Guo XH, Poloniecki JD, et al. . Initial energy setting, outcome and efficiency in direct current cardioversion of atrial fibrillation and flutter. J Am Coll Cardiol . 2001; 38:1498–504.CrossrefMedlineGoogle Scholar - 357.
Botkin SB, Dhanekula LS, Olshansky B . Outpatient cardioversion of atrial arrhythmias: efficacy, safety, and costs. Am Heart J . 2003; 145:233–8.CrossrefMedlineGoogle Scholar - 358.
Pizzale S, Lemery R, Green MS, et al. . Frequency and predictors of tachycardia-induced cardiomyopathy in patients with persistent atrial flutter. Can J Cardiol . 2009; 25:469–72.CrossrefMedlineGoogle Scholar - 359.
Van Gelder IC, Crijns HJ, Van Gilst WH, et al. . Prediction of uneventful cardioversion and maintenance of sinus rhythm from direct-current electrical cardioversion of chronic atrial fibrillation and flutter. Am J Cardiol . 1991; 68:41–6.CrossrefMedlineGoogle Scholar - 360.
Boriani G, Tukkie R, Manolis AS, et al. . Atrial antitachycardia pacing and managed ventricular pacing in bradycardia patients with paroxysmal or persistent atrial tachyarrhythmias: the MINERVA randomized multicentre international trial. Eur Heart J . 2014; 35:2352–62.CrossrefMedlineGoogle Scholar - 361.
Mitchell ARJ, Spurrell PAR, Cheatle L, et al. . Effect of atrial antitachycardia pacing treatments in patients with an atrial defibrillator: randomised study comparing subthreshold and nominal pacing outputs. Heart . 2002; 87:433–7.CrossrefMedlineGoogle Scholar - 362.
Gillis AM, Unterberg-Buchwald C, Schmidinger H, et al. . Safety and efficacy of advanced atrial pacing therapies for atrial tachyarrhythmias in patients with a new implantable dual chamber cardioverter-defibrillator. J Am Coll Cardiol . 2002; 40:1653–9.CrossrefMedlineGoogle Scholar - 363.
Waldo AL, MacLean WA, Cooper TB, et al. . Use of temporarily placed epicardial atrial wire electrodes for the diagnosis and treatment of cardiac arrhythmias following open-heart surgery. J Thorac Cardiovasc Surg . 1978; 76:500–5.CrossrefMedlineGoogle Scholar - 364.
Peters RW, Shorofsky SR, Pelini M, et al. . Overdrive atrial pacing for conversion of atrial flutter: comparison of postoperative with nonpostoperative patients. Am Heart J . 1999; 137:100–3.CrossrefMedlineGoogle Scholar - 365.
Ghali WA, Wasil BI, Brant R, et al. . Atrial flutter and the risk of thromboembolism: a systematic review and meta-analysis. Am J Med . 2005; 118:101–7.CrossrefMedlineGoogle Scholar - 366.
Hart RG, Pearce LA, Aguilar MI . Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med . 2007; 146:857–67.CrossrefMedlineGoogle Scholar - 367.
Gallagher MM, Hennessy BJ, Edvardsson N, et al. . Embolic complications of direct current cardioversion of atrial arrhythmias: association with low intensity of anticoagulation at the time of cardioversion. J Am Coll Cardiol . 2002; 40:926–33.CrossrefMedlineGoogle Scholar - 368.
Schmidt H, von der Recke G, Illien S, et al. . Prevalence of left atrial chamber and appendage thrombi in patients with atrial flutter and its clinical significance. J Am Coll Cardiol . 2001; 38:778–84.CrossrefMedlineGoogle Scholar - 369.
Lanzarotti CJ, Olshansky B . Thromboembolism in chronic atrial flutter: is the risk underestimated? J Am Coll Cardiol . 1997; 30:1506–11.CrossrefMedlineGoogle Scholar - 370.
Clemo HF, Wood MA, Gilligan DM, et al. . Intravenous amiodarone for acute heart rate control in the critically ill patient with atrial tachyarrhythmias. Am J Cardiol . 1998; 81:594–8.CrossrefMedlineGoogle Scholar - 371.
Roberts SA, Diaz C, Nolan PE, et al. . Effectiveness and costs of digoxin treatment for atrial fibrillation and flutter. Am J Cardiol . 1993; 72:567–73.CrossrefMedlineGoogle Scholar - 372.
Natale A, Newby KH, Pisanó E, et al. . Prospective randomized comparison of antiarrhythmic therapy versus first-line radiofrequency ablation in patients with atrial flutter. J Am Coll Cardiol . 2000; 35:1898–904.CrossrefMedlineGoogle Scholar - 373.
Bastani H, Drca N, Insulander P, et al. . Cryothermal vs. radiofrequency ablation as atrial flutter therapy: a randomized comparison. Europace . 2013; 15:420–8.CrossrefMedlineGoogle Scholar - 374.
Poty H, Saoudi N, Nair M, et al. . Radiofrequency catheter ablation of atrial flutter. Further insights into the various types of isthmus block: application to ablation during sinus rhythm. Circulation . 1996; 94:3204–13.CrossrefMedlineGoogle Scholar - 375.
Schwartzman D, Callans DJ, Gottlieb CD, et al. . Conduction block in the inferior vena caval-tricuspid valve isthmus: association with outcome of radiofrequency ablation of type I atrial flutter. J Am Coll Cardiol . 1996; 28:1519–31.CrossrefMedlineGoogle Scholar - 376.
Da Costa A, Thévenin J, Roche F, et al. . Results from the Loire-Ardèche-Drôme-Isère-Puy-de-Dôme (LADIP) trial on atrial flutter, a multicentric prospective randomized study comparing amiodarone and radiofrequency ablation after the first episode of symptomatic atrial flutter. Circulation . 2006; 114:1676–81.LinkGoogle Scholar - 377.
Pedersen OD, Bagger H, Keller N, et al. . Efficacy of dofetilide in the treatment of atrial fibrillation-flutter in patients with reduced left ventricular function: a Danish Investigations of Arrhythmia and Mortality on Dofetilide (DIAMOND) substudy. Circulation . 2001; 104:292–6.LinkGoogle Scholar - 378.
Benditt DG, Williams JH, Jin J, et al. . Maintenance of sinus rhythm with oral d,l-sotalol therapy in patients with symptomatic atrial fibrillation and/or atrial flutter. d,l-Sotalol Atrial Fibrillation/Flutter Study Group. Am J Cardiol . 1999; 84:270–7.CrossrefMedlineGoogle Scholar - 379.
Huang DT, Monahan KM, Zimetbaum P, et al. . Hybrid pharmacologic and ablative therapy: a novel and effective approach for the management of atrial fibrillation. J Cardiovasc Electrophysiol . 1998; 9:462–9.CrossrefMedlineGoogle Scholar - 380.
Schumacher B, Jung W, Lewalter T, et al. . Radiofrequency ablation of atrial flutter due to administration of class IC antiarrhythmic drugs for atrial fibrillation. Am J Cardiol . 1999; 83:710–3.CrossrefMedlineGoogle Scholar - 381.
Mohanty S, Mohanty P, Di Biase L, et al. . Results from a single-blind, randomized study comparing the impact of different ablation approaches on long-term procedure outcome in coexistent atrial fibrillation and flutter (APPROVAL). Circulation . 2013; 127:1853–60.LinkGoogle Scholar - 382.
Wazni O, Marrouche NF, Martin DO, et al. . Randomized study comparing combined pulmonary vein-left atrial junction disconnection and cavotricuspid isthmus ablation versus pulmonary vein-left atrial junction disconnection alone in patients presenting with typical atrial flutter and atrial fibrillation. Circulation . 2003; 108:2479–83.LinkGoogle Scholar - 383.
Aliot E, Denjoy I . Comparison of the safety and efficacy of flecainide versus propafenone in hospital out-patients with symptomatic paroxysmal atrial fibrillation/flutter. The Flecainide AF French Study Group. Am J Cardiol . 1996; 77:66A–71A.CrossrefMedlineGoogle Scholar - 384.
Van Gelder IC, Crijns HJ, Van Gilst WH, et al. . Efficacy and safety of flecainide acetate in the maintenance of sinus rhythm after electrical cardioversion of chronic atrial fibrillation or atrial flutter. Am J Cardiol . 1989; 64:1317–21.CrossrefMedlineGoogle Scholar - 385.
Pietersen AH, Hellemann H . Usefulness of flecainide for prevention of paroxysmal atrial fibrillation and flutter. Danish-Norwegian Flecainide Multicenter Study Group. Am J Cardiol . 1991; 67:713–7.CrossrefMedlineGoogle Scholar - 386.
Crijns HJ, Van Gelder IC, Kingma JH, et al. . Atrial flutter can be terminated by a class III antiarrhythmic drug but not by a class IC drug. Eur Heart J . 1994; 15:1403–8.CrossrefMedlineGoogle Scholar - 387.
Alboni P, Botto GL, Baldi N, et al. . Outpatient treatment of recent-onset atrial fibrillation with the "pill-in-the-pocket" approach. N Engl J Med . 2004; 351:2384–91.CrossrefMedlineGoogle Scholar - 388.
Ruder MA, Davis JC, Eldar M, et al. . Clinical and electrophysiologic characterization of automatic junctional tachycardia in adults. Circulation . 1986; 73:930–7.CrossrefMedlineGoogle Scholar - 389.
Villain E, Vetter VL, Garcia JM, et al. . Evolving concepts in the management of congenital junctional ectopic tachycardia. A multicenter study. Circulation . 1990; 81:1544–9.CrossrefMedlineGoogle Scholar - 390.
Raja P, Hawker RE, Chaikitpinyo A, et al. . Amiodarone management of junctional ectopic tachycardia after cardiac surgery in children. Br Heart J . 1994; 72:261–5.CrossrefMedlineGoogle Scholar - 391.
Wyndham CR, Dhingra RC, Smith T, et al. . Concealed nonparoxysmal junctional tachycardia. Circulation . 1979; 60:707–10.CrossrefMedlineGoogle Scholar - 392.
Konecke LL, Knoebel SB . Nonparoxysmal junctional tachycardia complicating acute myocardial infarction. Circulation . 1972; 45:367–74.CrossrefMedlineGoogle Scholar - 393.
Fishenfeld J, Desser KB, Benchimol A . Non-paroxysmal A-V junctional tachycardia associated with acute myocardial infarction. Am Heart J . 1973; 86:754–8.CrossrefMedlineGoogle Scholar - 394.
Breslow MJ, Evers AS, Lebowitz P . Successful treatment of accelerated junctional rhythm with propranolol: possible role of sympathetic stimulation in the genesis of this rhythm disturbance. Anesthesiology . 1985; 62:180–2.CrossrefMedlineGoogle Scholar - 395.
Lee KL, Chun HM, Liem LB, et al. . Effect of adenosine and verapamil in catecholamine-induced accelerated atrioventricular junctional rhythm: insights into the underlying mechanism. Pacing Clin Electrophysiol . 1999; 22:866–70.CrossrefMedlineGoogle Scholar - 396.
Padanilam BJ, Manfredi JA, Steinberg LA, et al. . Differentiating junctional tachycardia and atrioventricular node re-entry tachycardia based on response to atrial extrastimulus pacing. J Am Coll Cardiol . 2008; 52:1711–7.CrossrefMedlineGoogle Scholar - 397.
Kumagai K, Yamato H, Yamanouchi Y, et al. . Automatic junctional tachycardia in an adult. Clin Cardiol . 1990; 13:813–6.CrossrefMedlineGoogle Scholar - 398.
Cook JR, Steinberg JS . An incessant form of junctional ectopic tachycardia in an adult responsive to a class 1C agent. Am Heart J . 1991; 122:1487–9.CrossrefMedlineGoogle Scholar - 399.
Kuck KH, Kunze KP, Schlüter M, et al. . Encainide versus flecainide for chronic atrial and junctional ectopic tachycardia. Am J Cardiol . 1988; 62:37L–44L.CrossrefMedlineGoogle Scholar - 400.
Paul T, Reimer A, Janousek J, et al. . Efficacy and safety of propafenone in congenital junctional ectopic tachycardia. J Am Coll Cardiol . 1992; 20:911–4.CrossrefMedlineGoogle Scholar - 401.
Sarubbi B, Musto B, Ducceschi V, et al. . Congenital junctional ectopic tachycardia in children and adolescents: a 20 year experience based study. Heart . 2002; 88:188–90.CrossrefMedlineGoogle Scholar - 402.
Mamchur SE, Kurilin MY . High-amplitude pace mapping increases safety of radiofrequency catheter ablation of parahisian ectopic foci. Pacing Clin Electrophysiol . 2012; 35:1458–63.CrossrefMedlineGoogle Scholar - 403.
Scheinman MM, Gonzalez RP, Cooper MW, et al. . Clinical and electrophysiologic features and role of catheter ablation techniques in adult patients with automatic atrioventricular junctional tachycardia. Am J Cardiol . 1994; 74:565–72.CrossrefMedlineGoogle Scholar - 404.
Fan R, Tardos JG, Almasry I, et al. . Novel use of atrial overdrive pacing to rapidly differentiate junctional tachycardia from atrioventricular nodal reentrant tachycardia. Heart Rhythm . 2011; 8:840–4.CrossrefMedlineGoogle Scholar - 405.
Srivathsan K, Gami AS, Barrett R, et al. . Differentiating atrioventricular nodal reentrant tachycardia from junctional tachycardia: novel application of the delta H-A interval. J Cardiovasc Electrophysiol . 2008; 19:1–6.MedlineGoogle Scholar - 406.
Field ME, Miyazaki H, Epstein LM, et al. . Narrow complex tachycardia after slow pathway ablation: continue ablating? J Cardiovasc Electrophysiol . 2006; 17:557–9.CrossrefMedlineGoogle Scholar - 407.
Garson A, Gillette PC . Electrophysiologic studies of supraventricular tachycardia in children. I. Clinical-electrophysiologic correlations. Am Heart J . 1981; 102:233–50.CrossrefMedlineGoogle Scholar - 408.
Ko JK, Deal BJ, Strasburger JF, et al. . Supraventricular tachycardia mechanisms and their age distribution in pediatric patients. Am J Cardiol . 1992; 69:1028–32.CrossrefMedlineGoogle Scholar - 409.
Weindling SN, Saul JP, Walsh EP . Efficacy and risks of medical therapy for supraventricular tachycardia in neonates and infants. Am Heart J . 1996; 131:66–72.CrossrefMedlineGoogle Scholar - 410.
Garson A, Gillette PC, McNamara DG . Supraventricular tachycardia in children: clinical features, response to treatment, and long-term follow-up in 217 patients. J Pediatr . 1981; 98:875–82.CrossrefMedlineGoogle Scholar - 411.
Di Mambro C, Russo MS, Righi D, et al. . Ventricular pre-excitation: symptomatic and asymptomatic children have the same potential risk of sudden cardiac death. Europace . 2015:617–21.CrossrefMedlineGoogle Scholar - 412. Pediatric and Congenital Electrophysiology Society (PACES), Heart Rhythm Society (HRS), American College of Cardiology Foundation (ACCF), , et al.. PACES/HRS expert consensus statement on the management of the asymptomatic young patient with a Wolff-Parkinson-White (WPW, ventricular preexcitation) electrocardiographic pattern. Developed in partnership between the Pediatric and Congenital Electrophysiology Society (PACES) and the Heart Rhythm Society (HRS). Heart Rhythm . 2012; 9:1006–24.MedlineGoogle Scholar
- 413.
Dixon J, Foster K, Wyllie J, et al. . Guidelines and adenosine dosing in supraventricular tachycardia. Arch Dis Child . 2005; 90:1190–1.CrossrefMedlineGoogle Scholar - 414.
Díaz-Parra S, Sánchez-Yañez P, Zabala-Argüelles I, et al. . Use of adenosine in the treatment of supraventricular tachycardia in a pediatric emergency department. Pediatr Emerg Care . 2014; 30:388–93.CrossrefMedlineGoogle Scholar - 415.
Losek JD, Endom E, Dietrich A, et al. . Adenosine and pediatric supraventricular tachycardia in the emergency department: multicenter study and review. Ann Emerg Med . 1999; 33:185–91.CrossrefMedlineGoogle Scholar - 416.
Sanatani S, Potts JE, Reed JH, et al. . The study of antiarrhythmic medications in infancy (SAMIS): a multicenter, randomized controlled trial comparing the efficacy and safety of digoxin versus propranolol for prophylaxis of supraventricular tachycardia in infants. Circ Arrhythm Electrophysiol . 2012; 5:984–91.LinkGoogle Scholar - 417.
Deal BJ, Keane JF, Gillette PC, et al. . Wolff-Parkinson-White syndrome and supraventricular tachycardia during infancy: management and follow-up. J Am Coll Cardiol . 1985; 5:130–5.CrossrefMedlineGoogle Scholar - 418.
Villain E, Bonnet D, Acar P, et al. . [Recommendations for the treatment of recurrent supraventricular tachycardia in infants]. Arch Pediatr . 1998; 5:133–8.CrossrefMedlineGoogle Scholar - 419.
Fish FA, Gillette PC, Benson DW Proarrhythmia, cardiac arrest and death in young patients receiving encainide and flecainide. The Pediatric Electrophysiology Group. J Am Coll Cardiol . 1991; 18:356–65.CrossrefMedlineGoogle Scholar - 420.
Lee P-C, Hwang B, Chen S-A, et al. . The results of radiofrequency catheter ablation of supraventricular tachycardia in children. Pacing Clin Electrophysiol . 2007; 30:655–61.CrossrefMedlineGoogle Scholar - 421.
Van Hare GF, Javitz H, Carmelli D, et al. . Prospective assessment after pediatric cardiac ablation: demographics, medical profiles, and initial outcomes. J Cardiovasc Electrophysiol . 2004; 15:759–70.CrossrefMedlineGoogle Scholar - 422.
Aiyagari R, Saarel EV, Etheridge SP, et al. . Radiofrequency ablation for supraventricular tachycardia in children < or =15 kg is safe and effective. Pediatr Cardiol . 2005; 26:622–6.CrossrefMedlineGoogle Scholar - 423.
Blaufox AD, Felix GL, Saul JP, et al. . Radiofrequency catheter ablation in infants </=18 months old: when is it done and how do they fare?: Short-term data from the Pediatric Ablation Registry. Circulation . 2001; 104:2803–8.CrossrefMedlineGoogle Scholar - 424.
Blaufox AD, Paul T, Saul JP . Radiofrequency catheter ablation in small children: relationship of complications to application dose. Pacing Clin Electrophysiol . 2004; 27:224–9.CrossrefMedlineGoogle Scholar - 425.
Kantoch MJ, Gulamhusein SS, Sanatani S . Short- and long-term outcomes in children undergoing radiofrequency catheter ablation before their second birthday. Can J Cardiol . 2011; 27:523.e3–9.CrossrefGoogle Scholar - 426.
Schaffer MS, Gow RM, Moak JP, et al. . Mortality following radiofrequency catheter ablation (from the Pediatric Radiofrequency Ablation Registry). Participating members of the Pediatric Electrophysiology Society. Am J Cardiol . 2000; 86:639–43.CrossrefMedlineGoogle Scholar - 427.
Van Hare GF, Javitz H, Carmelli D, et al. . Prospective assessment after pediatric cardiac ablation: recurrence at 1 year after initially successful ablation of supraventricular tachycardia. Heart Rhythm . 2004; 1:188–96.CrossrefMedlineGoogle Scholar - 428.
Buddhe S, Singh H, Du W, et al. . Radiofrequency and cryoablation therapies for supraventricular arrhythmias in the young: five-year review of efficacies. Pacing Clin Electrophysiol . 2012; 35:711–7.CrossrefMedlineGoogle Scholar - 429.
Chiu S-N, Lu C-W, Chang C-W, et al. . Radiofrequency catheter ablation of supraventricular tachycardia in infants and toddlers. Circ J . 2009; 73:1717–21.CrossrefMedlineGoogle Scholar - 430.
Batra AS, Chun DS, Johnson TR, et al. . A prospective analysis of the incidence and risk factors associated with junctional ectopic tachycardia following surgery for congenital heart disease. Pediatr Cardiol . 2006; 27:51–5.CrossrefMedlineGoogle Scholar - 431.
Andreasen JB, Johnsen SP, Ravn HB . Junctional ectopic tachycardia after surgery for congenital heart disease in children. Intensive Care Med . 2008; 34:895–902.CrossrefMedlineGoogle Scholar - 432.
Haas NA, Camphausen CK . Impact of early and standardized treatment with amiodarone on therapeutic success and outcome in pediatric patients with postoperative tachyarrhythmia. J Thorac Cardiovasc Surg . 2008; 136:1215–22.CrossrefMedlineGoogle Scholar - 433.
Laird WP, Snyder CS, Kertesz NJ, et al. . Use of intravenous amiodarone for postoperative junctional ectopic tachycardia in children. Pediatr Cardiol . 2003; 24:133–7.CrossrefMedlineGoogle Scholar - 434.
Kovacikova L, Hakacova N, Dobos D, et al. . Amiodarone as a first-line therapy for postoperative junctional ectopic tachycardia. Ann Thorac Surg . 2009; 88:616–22.CrossrefMedlineGoogle Scholar - 435.
Walsh EP, Saul JP, Sholler GF, et al. . Evaluation of a staged treatment protocol for rapid automatic junctional tachycardia after operation for congenital heart disease. J Am Coll Cardiol . 1997; 29:1046–53.CrossrefMedlineGoogle Scholar - 436.
van Engelen AD, Weijtens O, Brenner JI, et al. . Management outcome and follow-up of fetal tachycardia. J Am Coll Cardiol . 1994; 24:1371–5.CrossrefMedlineGoogle Scholar - 437.
Jaeggi ET, Carvalho JS, De Groot E, et al. . Comparison of transplacental treatment of fetal supraventricular tachyarrhythmias with digoxin, flecainide, and sotalol: results of a nonrandomized multicenter study. Circulation . 2011; 124:1747–54.LinkGoogle Scholar - 438.
van der Heijden LB, Oudijk MA, Manten GTR, et al. . Sotalol as first-line treatment for fetal tachycardia and neonatal follow-up. Ultrasound Obstet Gynecol . 2013; 42:285–93.CrossrefMedlineGoogle Scholar - 439.
Strasburger JF, Cuneo BF, Michon MM, et al. . Amiodarone therapy for drug-refractory fetal tachycardia. Circulation . 2004; 109:375–9.LinkGoogle Scholar - 440.
Bouchardy J, Therrien J, Pilote L, et al. . Atrial arrhythmias in adults with congenital heart disease. Circulation . 2009; 120:1679–86.LinkGoogle Scholar - 441.
Bernier M, Marelli AJ, Pilote L, et al. . Atrial arrhythmias in adult patients with right- versus left-sided congenital heart disease anomalies. Am J Cardiol . 2010; 106:547–51.CrossrefMedlineGoogle Scholar - 442.
Engelfriet P, Boersma E, Oechslin E, et al. . The spectrum of adult congenital heart disease in Europe: morbidity and mortality in a 5 year follow-up period. The Euro Heart Survey on Adult Congenital Heart Disease. Eur Heart J . 2005; 26:2325–33.CrossrefMedlineGoogle Scholar - 443.
Trojnarska O, Grajek S, Kramer L, et al. . Risk factors of supraventricular arrhythmia in adults with congenital heart disease. Cardiol J . 2009; 16:218–26.MedlineGoogle Scholar - 444.
Verheugt CL, Uiterwaal CSPM, van der Velde ET, et al. . Gender and outcome in adult congenital heart disease. Circulation . 2008; 118:26–32.LinkGoogle Scholar - 445.
Collins KK, Love BA, Walsh EP, et al. . Location of acutely successful radiofrequency catheter ablation of intraatrial reentrant tachycardia in patients with congenital heart disease. Am J Cardiol . 2000; 86:969–74.CrossrefMedlineGoogle Scholar - 446.
de Groot NMS, Zeppenfeld K, Wijffels MC, et al. . Ablation of focal atrial arrhythmia in patients with congenital heart defects after surgery: role of circumscribed areas with heterogeneous conduction. Heart Rhythm . 2006; 3:526–35.CrossrefMedlineGoogle Scholar - 447.
Koyak Z, Kroon B, de Groot JR, et al. . Efficacy of antiarrhythmic drugs in adults with congenital heart disease and supraventricular tachycardias. Am J Cardiol . 2013; 112:1461–7.CrossrefMedlineGoogle Scholar - 448.
Mah DY, Alexander ME, Cecchin F, et al. . The electroanatomic mechanisms of atrial tachycardia in patients with tetralogy of Fallot and double outlet right ventricle. J Cardiovasc Electrophysiol . 2011; 22:1013–7.CrossrefMedlineGoogle Scholar - 449.
Wasmer K, Köbe J, Dechering DG, et al. . Isthmus-dependent right atrial flutter as the leading cause of atrial tachycardias after surgical atrial septal defect repair. Int J Cardiol . 2013; 168:2447–52.CrossrefMedlineGoogle Scholar - 450.
Cuypers JAAE, Opić P, Menting ME, et al. . The unnatural history of an atrial septal defect: longitudinal 35 year follow up after surgical closure at young age. Heart . 2013; 99:1346–52.CrossrefMedlineGoogle Scholar - 451.
Khairy P, Aboulhosn J, Gurvitz MZ, et al. . Arrhythmia burden in adults with surgically repaired tetralogy of Fallot: a multi-institutional study. Circulation . 2010; 122:868–75.LinkGoogle Scholar - 452.
de Groot NMS, Atary JZ, Blom NA, et al. . Long-term outcome after ablative therapy of postoperative atrial tachyarrhythmia in patients with congenital heart disease and characteristics of atrial tachyarrhythmia recurrences. Circ Arrhythm Electrophysiol . 2010; 3:148–54.LinkGoogle Scholar - 453.
Hårdhammar PA, van Beusekom HM, Emanuelsson HU, et al. . Reduction in thrombotic events with heparin-coated Palmaz-Schatz stents in normal porcine coronary arteries. Circulation . 1996; 93:423–30.CrossrefMedlineGoogle Scholar - 454.
Tanner H, Lukac P, Schwick N, et al. . Irrigated-tip catheter ablation of intraatrial reentrant tachycardia in patients late after surgery of congenital heart disease. Heart Rhythm . 2004; 1:268–75.CrossrefMedlineGoogle Scholar - 455.
Triedman JK, Saul JP, Weindling SN, et al. . Radiofrequency ablation of intra-atrial reentrant tachycardia after surgical palliation of congenital heart disease. Circulation . 1995; 91:707–14.CrossrefMedlineGoogle Scholar - 456.
DeMazumder D, Lake DE, Cheng A, et al. . Dynamic analysis of cardiac rhythms for discriminating atrial fibrillation from lethal ventricular arrhythmias. Circ Arrhythm Electrophysiol . 2013; 6:555–61.LinkGoogle Scholar - 457.
Yap S-C, Harris L, Silversides CK, et al. . Outcome of intra-atrial re-entrant tachycardia catheter ablation in adults with congenital heart disease: negative impact of age and complex atrial surgery. J Am Coll Cardiol . 2010; 56:1589–96.CrossrefMedlineGoogle Scholar - 458.
Scaglione M, Caponi D, Ebrille E, et al. . Very long-term results of electroanatomic-guided radiofrequency ablation of atrial arrhythmias in patients with surgically corrected atrial septal defect. Europace . 2014:1800–7.CrossrefMedlineGoogle Scholar - 459.
Feltes TF, Friedman RA . Transesophageal echocardiographic detection of atrial thrombi in patients with nonfibrillation atrial tachyarrhythmias and congenital heart disease. J Am Coll Cardiol . 1994; 24:1365–70.CrossrefMedlineGoogle Scholar - 460.
Ammash NM, Phillips SD, Hodge DO, et al. . Outcome of direct current cardioversion for atrial arrhythmias in adults with congenital heart disease. Int J Cardiol . 2012; 154:270–4.CrossrefMedlineGoogle Scholar - 461.
Pass RH, Liberman L, Al-Fayaddh M, et al. . Continuous intravenous diltiazem infusion for short-term ventricular rate control in children. Am J Cardiol . 2000; 86:559–562. A9.CrossrefMedlineGoogle Scholar - 462.
Garnock-Jones KP . Esmolol: a review of its use in the short-term treatment of tachyarrhythmias and the short-term control of tachycardia and hypertension. Drugs . 2012; 72:109–32.CrossrefMedlineGoogle Scholar - 463.
diMarco JP, Sellers TD, Lerman BB, et al. . Diagnostic and therapeutic use of adenosine in patients with supraventricular tachyarrhythmias. J Am Coll Cardiol . 1985; 6:417–25.CrossrefMedlineGoogle Scholar - 464.
Haines DE, DiMarco JP . Sustained intraatrial reentrant tachycardia: clinical, electrocardiographic and electrophysiologic characteristics and long-term follow-up. J Am Coll Cardiol . 1990; 15:1345–54.CrossrefMedlineGoogle Scholar - 465.
Kall JG, Kopp D, Olshansky B, et al. . Adenosine-sensitive atrial tachycardia. Pacing Clin Electrophysiol . 1995; 18:300–6.CrossrefMedlineGoogle Scholar - 466.
Olshansky B, Okumura K, Hess PG, et al. . Use of procainamide with rapid atrial pacing for successful conversion of atrial flutter to sinus rhythm. J Am Coll Cardiol . 1988; 11:359–64.CrossrefMedlineGoogle Scholar - 467.
Heisel A, Jung J, Stopp M, et al. . Facilitating influence of procainamide on conversion of atrial flutter by rapid atrial pacing. Eur Heart J . 1997; 18:866–9.CrossrefMedlineGoogle Scholar - 468.
Hoyer AW, Balaji S . The safety and efficacy of ibutilide in children and in patients with congenital heart disease. Pacing Clin Electrophysiol . 2007; 30:1003–8.CrossrefMedlineGoogle Scholar - 469.
Crawford W, Plumb VJ, Epstein AE, et al. . Prospective evaluation of transesophageal pacing for the interruption of atrial flutter. Am J Med . 1989; 86:663–7.CrossrefMedlineGoogle Scholar - 470.
Rhodes LA, Walsh EP, Saul JP . Conversion of atrial flutter in pediatric patients by transesophageal atrial pacing: a safe, effective, minimally invasive procedure. Am Heart J . 1995; 130:323–7.CrossrefMedlineGoogle Scholar - 471.
Brockmeier K, Ulmer HE, Hessling G . Termination of atrial reentrant tachycardias by using transesophageal atrial pacing. J Electrocardiol . 2002; 35Suppl:159–63.CrossrefMedlineGoogle Scholar - 472.
Stephenson EA, Casavant D, Tuzi J, et al. . Efficacy of atrial antitachycardia pacing using the Medtronic AT500 pacemaker in patients with congenital heart disease. Am J Cardiol . 2003; 92:871–6.CrossrefMedlineGoogle Scholar - 473.
Rao SO, Boramanand NK, Burton DA, et al. . Atrial tachycardias in young adults and adolescents with congenital heart disease: conversion using single dose oral sotalol. Int J Cardiol . 2009; 136:253–7.CrossrefMedlineGoogle Scholar - 474.
Wells R, Khairy P, Harris L, et al. . Dofetilide for atrial arrhythmias in congenital heart disease: a multicenter study. Pacing Clin Electrophysiol . 2009; 32:1313–8.CrossrefMedlineGoogle Scholar - 475.
Murphy JG, Gersh BJ, McGoon MD, et al. . Long-term outcome after surgical repair of isolated atrial septal defect. Follow-up at 27 to 32 years. N Engl J Med . 1990; 323:1645–50.CrossrefMedlineGoogle Scholar - 476.
Konstantinides S, Geibel A, Olschewski M, et al. . A comparison of surgical and medical therapy for atrial septal defect in adults. N Engl J Med . 1995; 333:469–73.CrossrefMedlineGoogle Scholar - 477.
Pfammatter JP, Paul T, Ziemer G, et al. . Successful management of junctional tachycardia by hypothermia after cardiac operations in infants. Ann Thorac Surg . 1995; 60:556–60.CrossrefMedlineGoogle Scholar - 478.
Giamberti A, Chessa M, Foresti S, et al. . Combined atrial septal defect surgical closure and irrigated radiofrequency ablation in adult patients. Ann Thorac Surg . 2006; 82:1327–31.CrossrefMedlineGoogle Scholar - 479.
Huang CJ, Chiu IS, Lin FY, et al. . Role of electrophysiological studies and arrhythmia intervention in repairing Ebstein's anomaly. Thorac Cardiovasc Surg . 2000; 48:347–50.CrossrefMedlineGoogle Scholar - 480.
Reich JD, Auld D, Hulse E, et al. . The Pediatric Radiofrequency Ablation Registry's experience with Ebstein's anomaly. Pediatric Electrophysiology Society. J Cardiovasc Electrophysiol . 1998; 9:1370–7.CrossrefMedlineGoogle Scholar - 481.
Cappato R, Schlüter M, Weiss C, et al. . Radiofrequency current catheter ablation of accessory atrioventricular pathways in Ebstein's anomaly. Circulation . 1996; 94:376–83.LinkGoogle Scholar - 482.
Misaki T, Watanabe G, Iwa T, et al. . Surgical treatment of patients with Wolff-Parkinson-White syndrome and associated Ebstein's anomaly. J Thorac Cardiovasc Surg . 1995; 110:1702–7.CrossrefMedlineGoogle Scholar - 483.
Khositseth A, Danielson GK, Dearani JA, et al. . Supraventricular tachyarrhythmias in Ebstein anomaly: management and outcome. J Thorac Cardiovasc Surg . 2004; 128:826–33.CrossrefMedlineGoogle Scholar - 484.
Bockeria L, Golukhova E, Dadasheva M, et al. . Advantages and disadvantages of one-stage and two-stage surgery for arrhythmias and Ebstein's anomaly. Eur J Cardiothorac Surg . 2005; 28:536–40.CrossrefMedlineGoogle Scholar - 485.
Pressley JC, Wharton JM, Tang AS, et al. . Effect of Ebstein's anomaly on short- and long-term outcome of surgically treated patients with Wolff-Parkinson-White syndrome. Circulation . 1992; 86:1147–55.CrossrefMedlineGoogle Scholar - 486.
Khairy P, Harris L, Landzberg MJ, et al. . Sudden death and defibrillators in transposition of the great arteries with intra-atrial baffles: a multicenter study. Circ Arrhythm Electrophysiol . 2008; 1:250–7.LinkGoogle Scholar - 487.
Banchs JE, Baquero GA, Nickolaus MJ, et al. . Clinical efficacy of dofetilide for the treatment of atrial tachyarrhythmias in adults with congenital heart disease. Congenit Heart Dis . 2014; 9:221–7.CrossrefMedlineGoogle Scholar - 488.
Tanel RE, Walsh EP, Lulu JA, et al. . Sotalol for refractory arrhythmias in pediatric and young adult patients: initial efficacy and long-term outcome. Am Heart J . 1995; 130:791–7.CrossrefMedlineGoogle Scholar - 489.
Lukac P, Pedersen AK, Mortensen PT, et al. . Ablation of atrial tachycardia after surgery for congenital and acquired heart disease using an electroanatomic mapping system: which circuits to expect in which substrate? Heart Rhythm . 2005; 2:64–72.CrossrefMedlineGoogle Scholar - 490.
Yap S-C, Harris L, Downar E, et al. . Evolving electroanatomic substrate and intra-atrial reentrant tachycardia late after Fontan surgery. J Cardiovasc Electrophysiol . 2012; 23:339–45.CrossrefMedlineGoogle Scholar - 491.
Wu J, Deisenhofer I, Ammar S, et al. . Acute and long-term outcome after catheter ablation of supraventricular tachycardia in patients after the Mustard or Senning operation for D-transposition of the great arteries. Europace . 2013; 15:886–91.CrossrefMedlineGoogle Scholar - 492.
Jones DG, Jarman JWE, Lyne JC, et al. . The safety and efficacy of trans-baffle puncture to enable catheter ablation of atrial tachycardias following the Mustard procedure: a single centre experience and literature review. Int J Cardiol . 2013; 168:1115–20.CrossrefMedlineGoogle Scholar - 493.
Kalman JM, VanHare GF, Olgin JE, et al. . Ablation of 'incisional' reentrant atrial tachycardia complicating surgery for congenital heart disease. Use of entrainment to define a critical isthmus of conduction. Circulation . 1996; 93:502–12.CrossrefMedlineGoogle Scholar - 494.
Ueda A, Suman-Horduna I, Mantziari L, et al. . Contemporary outcomes of supraventricular tachycardia ablation in congenital heart disease: a single-center experience in 116 patients. Circ Arrhythm Electrophysiol . 2013; 6:606–13.LinkGoogle Scholar - 495.
de Groot NMS, Lukac P, Blom NA, et al. . Long-term outcome of ablative therapy of postoperative supraventricular tachycardias in patients with univentricular heart: a European multicenter study. Circ Arrhythm Electrophysiol . 2009; 2:242–8.LinkGoogle Scholar - 496.
Silversides CK, Salehian O, Oechslin E, et al. . Canadian Cardiovascular Society 2009 Consensus Conference on the management of adults with congenital heart disease: complex congenital cardiac lesions. Can J Cardiol . 2010; 26:e98–117.CrossrefMedlineGoogle Scholar - 497.
Giamberti A, Chessa M, Abella R, et al. . Surgical treatment of arrhythmias in adults with congenital heart defects. Int J Cardiol . 2008; 129:37–41.CrossrefMedlineGoogle Scholar - 498.
Stulak JM, Dearani JA, Puga FJ, et al. . Right-sided Maze procedure for atrial tachyarrhythmias in congenital heart disease. Ann Thorac Surg . 2006; 81:1780–4.CrossrefMedlineGoogle Scholar - 499.
Mavroudis C, Deal BJ, Backer CL, et al. . Arrhythmia surgery in patients with and without congenital heart disease. Ann Thorac Surg . 2008; 86:857–68.CrossrefMedlineGoogle Scholar - 500.
Karamlou T, Silber I, Lao R, et al. . Outcomes after late reoperation in patients with repaired tetralogy of Fallot: the impact of arrhythmia and arrhythmia surgery. Ann Thorac Surg . 2006; 81:1786–93.CrossrefMedlineGoogle Scholar - 501.
Deal BJ, Mavroudis C, Backer CL, et al. . Comparison of anatomic isthmus block with the modified right atrial maze procedure for late atrial tachycardia in Fontan patients. Circulation . 2002; 106:575–9.LinkGoogle Scholar - 502.
Mavroudis C, Backer CL, Deal BJ, et al. . Total cavopulmonary conversion and maze procedure for patients with failure of the Fontan operation. J Thorac Cardiovasc Surg . 2001; 122:863–71.CrossrefMedlineGoogle Scholar - 503.
Mavroudis C, Deal BJ, Backer CL, et al. . J. Maxwell Chamberlain Memorial Paper for congenital heart surgery. 111 Fontan conversions with arrhythmia surgery: surgical lessons and outcomes. Ann Thorac Surg . 2007; 84:1457–65.CrossrefMedlineGoogle Scholar - 504.
Aboulhosn J, Williams R, Shivkumar K, et al. . Arrhythmia recurrence in adult patients with single ventricle physiology following surgical Fontan conversion. Congenit Heart Dis . 2010; 5:430–4.CrossrefMedlineGoogle Scholar - 505.
Said SM, Burkhart HM, Schaff HV, et al. . Fontan conversion: identifying the high-risk patient. Ann Thorac Surg . 2014; 97:2115–21.CrossrefMedlineGoogle Scholar - 506.
Gutierrez SD, Earing MG, Singh AK, et al. . Atrial tachyarrhythmias and the Cox-maze procedure in congenital heart disease. Congenit Heart Dis . 2013; 8:434–9.MedlineGoogle Scholar - 507.
Terada T, Sakurai H, Nonaka T, et al. . Surgical outcome of Fontan conversion and arrhythmia surgery: need a pacemaker? Asian Cardiovasc Thorac Ann . 2013; 22:682–6.CrossrefMedlineGoogle Scholar - 508.
Theodoro DA, Danielson GK, Porter CJ, et al. . Right-sided maze procedure for right atrial arrhythmias in congenital heart disease. Ann Thorac Surg . 1998; 65:149–53.CrossrefMedlineGoogle Scholar - 509.
Silka MJ, Manwill JR, Kron J, et al. . Bradycardia-mediated tachyarrhythmias in congenital heart disease and responses to chronic pacing at physiologic rates. Am J Cardiol . 1990; 65:488–93.CrossrefMedlineGoogle Scholar - 510.
Ragonese P, Drago F, Guccione P, et al. . Permanent overdrive atrial pacing in the chronic management of recurrent postoperative atrial reentrant tachycardia in patients with complex congenital heart disease. Pacing Clin Electrophysiol . 1997; 20:2917–23.CrossrefMedlineGoogle Scholar - 511.
Thorne SA, Barnes I, Cullinan P, et al. . Amiodarone-associated thyroid dysfunction: risk factors in adults with congenital heart disease. Circulation . 1999; 100:149–54.CrossrefMedlineGoogle Scholar - 512.
Stan MN, Hess EP, Bahn RS, et al. . A risk prediction index for amiodarone-induced thyrotoxicosis in adults with congenital heart disease. J Thyroid Res . 2012; 2012:210529.CrossrefMedlineGoogle Scholar - 513.
Shotan A, Ostrzega E, Mehra A, et al. . Incidence of arrhythmias in normal pregnancy and relation to palpitations, dizziness, and syncope. Am J Cardiol . 1997; 79:1061–4.CrossrefMedlineGoogle Scholar - 514.
Tawam M, Levine J, Mendelson M, et al. . Effect of pregnancy on paroxysmal supraventricular tachycardia. Am J Cardiol . 1993; 72:838–40.CrossrefMedlineGoogle Scholar - 515.
Silversides CK, Harris L, Haberer K, et al. . Recurrence rates of arrhythmias during pregnancy in women with previous tachyarrhythmia and impact on fetal and neonatal outcomes. Am J Cardiol . 2006; 97:1206–12.CrossrefMedlineGoogle Scholar - 516.
Ghosh N, Luk A, Derzko C, et al. . The acute treatment of maternal supraventricular tachycardias during pregnancy: a review of the literature. J Obstet Gynaecol Can . 2011; 33:17–23.CrossrefMedlineGoogle Scholar - 517.
Qasqas SA, McPherson C, Frishman WH, et al. . Cardiovascular pharmacotherapeutic considerations during pregnancy and lactation. Cardiol Rev . 2004; 12:201–21.CrossrefMedlineGoogle Scholar - 518.
Page RL . Treatment of arrhythmias during pregnancy. Am Heart J . 1995; 130:871–6.CrossrefMedlineGoogle Scholar - 519.
Tromp CHN, Nanne ACM, Pernet PJM, et al. . Electrical cardioversion during pregnancy: safe or not? Neth Heart J . 2011; 19:134–6.CrossrefMedlineGoogle Scholar - 520.
Vanden Hoek TL, Morrison LJ, Shuster M, et al. . Part 12: cardiac arrest in special situations: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation . 2010; 122:S829–61.LinkGoogle Scholar - 521.
Ersbøll AS, Hedegaard M, Søndergaard L, et al. . Treatment with oral beta-blockers during pregnancy complicated by maternal heart disease increases the risk of fetal growth restriction. BJOG . 2014; 121:618–26.CrossrefMedlineGoogle Scholar - 522.
Allen NM, Page RL . Procainamide administration during pregnancy. Clin Pharm . 1993; 12:58–60.MedlineGoogle Scholar - 523.
Bartalena L, Bogazzi F, Braverman LE, et al. . Effects of amiodarone administration during pregnancy on neonatal thyroid function and subsequent neurodevelopment. J Endocrinol Invest . 2001; 24:116–30.CrossrefMedlineGoogle Scholar - 524.
Meidahl Petersen K, Jimenez-Solem E, Andersen JT, et al. . ß-Blocker treatment during pregnancy and adverse pregnancy outcomes: a nationwide population-based cohort study. BMJ Open . 2012;2.Google Scholar - 525.
Lydakis C, Lip GY, Beevers M, et al. . Atenolol and fetal growth in pregnancies complicated by hypertension. Am J Hypertens . 1999; 12:541–7.CrossrefMedlineGoogle Scholar - 526.
Szumowski L, Szufladowicz E, Orczykowski M, et al. . Ablation of severe drug-resistant tachyarrhythmia during pregnancy. J Cardiovasc Electrophysiol . 2010; 21:877–82.MedlineGoogle Scholar - 527.
Damilakis J, Theocharopoulos N, Perisinakis K, et al. . Conceptus radiation dose and risk from cardiac catheter ablation procedures. Circulation . 2001; 104:893–7.CrossrefMedlineGoogle Scholar - 528.
McCollough CH, Schueler BA, Atwell TD, et al. . Radiation exposure and pregnancy: when should we be concerned? Radiographics . 2007; 27:909–17.CrossrefMedlineGoogle Scholar - 529.
Colletti PM, Lee KH, Elkayam U . Cardiovascular imaging of the pregnant patient. AJR Am J Roentgenol . 2013; 200:515–21.CrossrefMedlineGoogle Scholar - 530.
Chen SA, Lee SH, Wu TJ, et al. . Initial onset of accessory pathway-mediated and atrioventricular node reentrant tachycardia after age 65: clinical features, electrophysiologic characteristics, and possible facilitating factors. J Am Geriatr Soc . 1995; 43:1370–7.CrossrefMedlineGoogle Scholar - 531.
Chen SA, Chiang CE, Tai CT, et al. . Complications of diagnostic electrophysiologic studies and radiofrequency catheter ablation in patients with tachyarrhythmias: an eight-year survey of 3,966 consecutive procedures in a tertiary referral center. Am J Cardiol . 1996; 77:41–6.CrossrefMedlineGoogle Scholar - 532.
Zado ES, Callans DJ, Gottlieb CD, et al. . Efficacy and safety of catheter ablation in octogenarians. J Am Coll Cardiol . 2000; 35:458–62.CrossrefMedlineGoogle Scholar - 533.
Rostock T, Risius T, Ventura R, et al. . Efficacy and safety of radiofrequency catheter ablation of atrioventricular nodal reentrant tachycardia in the elderly. J Cardiovasc Electrophysiol . 2005; 16:608–10.CrossrefMedlineGoogle Scholar - 534.
Kihel J, Da Costa A, Kihel A, et al. . Long-term efficacy and safety of radiofrequency ablation in elderly patients with atrioventricular nodal re-entrant tachycardia. Europace . 2006; 8:416–20.CrossrefMedlineGoogle Scholar - 535.
Dagres N, Piorkowski C, Kottkamp H, et al. . Contemporary catheter ablation of arrhythmias in geriatric patients: patient characteristics, distribution of arrhythmias, and outcome. Europace . 2007; 9:477–80.CrossrefMedlineGoogle Scholar - 536.
Pedrinazzi C, Durin O, Agricola P, et al. . Efficacy and safety of radiofrequency catheter ablation in the elderly. J Interv Card Electrophysiol . 2007; 19:179–85.CrossrefMedlineGoogle Scholar - 537.
Hoffmann BA, Brachmann J, Andresen D, et al. . Ablation of atrioventricular nodal reentrant tachycardia in the elderly: results from the German Ablation Registry. Heart Rhythm . 2011; 8:981–7.CrossrefMedlineGoogle Scholar - 538.
Yangni N'Da' O, Brembilla-Perrot B . Clinical characteristics and management of paroxysmal junctional tachycardia in the elderly. Arch Cardiovasc Dis . 2008; 101:143–8.CrossrefMedlineGoogle Scholar - 539.
Boulos M, Hoch D, Schecter S, et al. . Age dependence of complete heart block complicating radiofrequency ablation of the atrioventricular nodal slow pathway. Am J Cardiol . 1998; 82:390–1.CrossrefMedlineGoogle Scholar - 540.
Goldberg AS, Bathina MN, Mickelsen S, et al. . Long-term outcomes on quality-of-life and health care costs in patients with supraventricular tachycardia (radiofrequency catheter ablation versus medical therapy). Am J Cardiol . 2002; 89:1120–3.CrossrefMedlineGoogle Scholar - 541.
Bathina MN, Mickelsen S, Brooks C, et al. . Radiofrequency catheter ablation versus medical therapy for initial treatment of supraventricular tachycardia and its impact on quality of life and healthcare costs. Am J Cardiol . 1998; 82:589–93.CrossrefMedlineGoogle Scholar - 542.
Meissner A, Stifoudi I, Weismüller P, et al. . Sustained high quality of life in a 5-year long term follow-up after successful ablation for supra- ventricular tachycardia: results from a large retrospective patient cohort. Int J Med Sci . 2009; 6:28–36.CrossrefMedlineGoogle Scholar - 543.
Wood KA, Stewart AL, Drew BJ, et al. . Patient perception of symptoms and quality of life following ablation in patients with supraventricular tachycardia. Heart Lung . 2010; 39:12–20.CrossrefMedlineGoogle Scholar - 544.
Yildirim O, Yontar OC, Semiz M, et al. . The effect of radiofrequency ablation treatment on quality of life and anxiety in patients with supraventricular tachycardia. Eur Rev Med Pharmacol Sci . 2012; 16:2108–12.MedlineGoogle Scholar - 545.
Farkowski MM, Pytkowski M, Maciag A, et al. . Gender-related differences in outcomes and resource utilization in patients undergoing radiofrequency ablation of supraventricular tachycardia: results from Patients' Perspective on Radiofrequency Catheter Ablation of AVRT and AVNRT Study. Europace . 2014:1821–7.CrossrefMedlineGoogle Scholar - 546.
Larson MS, McDonald K, Young C, et al. . Quality of life before and after radiofrequency catheter ablation in patients with drug refractory atrioventricular nodal reentrant tachycardia. Am J Cardiol . 1999; 84:471–3, A9.CrossrefGoogle Scholar - 547.
Dewland TA, Glidden DV, Marcus GM . Healthcare utilization and clinical outcomes after catheter ablation of atrial flutter. PLoS One . 2014; 9:e100509.CrossrefMedlineGoogle Scholar
| Committee Member | Employment | Consultant | Speakers Bureau | Ownership/Partnership/Principal | Personal Research | Institutional, Organizational, or Other Financial Benefit | Expert Witness | Voting Recusals by Section* |
|---|---|---|---|---|---|---|---|---|
| Richard L. Page, Chair | University of Wisconsin School of Medicine and Public Health—Chair, Department of Medicine | None | None | None | None | None | None | None |
| José A. Joglar, Vice Chair | University of Texas Southwestern Medical Center—Professor of Internal Medicine; Program Director, Clinical Cardiac Electrophysiology | None | None | None | None | None | None | None |
| Sana M. Al-Khatib | Duke Clinical Research Institute—Associate Professor of Medicine | None | None | None | None | None | None | None |
| Mary A. Caldwell | University of California San Francisco—Assistant Professor (Retired) | None | None | None | None | None | None | None |
| Hugh Calkins | Johns Hopkins Hospital—Professor of Medicine, Director of Electrophysiology | • Atricure• Boehringer Ingelheim• Daiichi-Sankyo | None | None | • St. Jude Medical† | None | None | All Sections except 2.4, 5.2, 6.1.2, 9.3.2, and 9.4. |
| Jamie B. Conti | University of Florida—Professor of Medicine, Chief of Cardiovascular Medicine | None | None | None | • Medtronic | • Boston Scientific‡• Medtronic‡• St. Jude Medical‡ | None | All Sections except 2.4, 6.1.2, 9.3.2, and 9.4. |
| Barbara J. Deal | Feinberg School of Medicine, Northwestern University—Professor of Pediatrics; Ann & Robert H. Lurie Children's Hospital of Chicago—Division Head, Cardiology | None | None | None | None | None | None | None |
| N.A. Mark Estes III | Tufts University School of Medicine—Professor of Medicine | • Boston Scientific†• Medtronic• St. Jude Medical | None | None | • Boston Scientific | • Boston Scientific†• Medtronic†• St. Jude Medical† | None | All Sections except 2.4, 5.2, 6.1.2, 9.3.2, and 9.4. |
| Michael E. Field | University of Wisconsin School of Medicine and Public Health—Assistant Professor of Medicine, Director of Cardiac Arrhythmia Service | None | None | None | None | None | None | None |
| Zachary D. Goldberger | University of Washington School of Medicine—Assistant Professor of Medicine | None | None | None | None | None | None | None |
| Stephen C. Hammill | Mayo Clinic—Professor Emeritus of Medicine | None | None | None | None | None | None | None |
| Julia H. Indik | University of Arizona—Associate Professor of Medicine | None | None | None | None | None | None | None |
| Bruce D. Lindsay | Cleveland Clinic Foundation—Professor of Cardiology | • Biosense Webster• Boston Scientific• CardioInsight• Medtronic | None | None | None | • Boston Scientific†• Medtronic†• St. Jude Medical† | None | All Sections except 2.4, 5.2, 6.1.2, 9.3.2, and 9.4. |
| Brian Olshansky | University of Iowa Hospitals—Professor Emeritus of Medicine; Mercy Hospital Mason City—Electrophysiologist | • BioControl• Biotronik• Boehringer-Ingelheim• Boston Scientific-Guidant• Daiichi-Sankyo• Medtronic†• Sanofi-aventis | None | None | • Amarin (DSMB)• Boston Scientific (DSMB)• Sanofi-aventis (DSMB) | • Boston Scientific | None | All Sections except 2.4 and 9.4. |
| Andrea M. Russo | Cooper Medical School of Rowan University—Professor of Medicine; Cooper University Hospital—Director, Electrophysiology and Arrhythmia Services | • Biotronik• Boston Scientific• Medtronic• St. Jude Medical | None | None | • Medtronic† | • Biotronik‡• Boston Scientific† | None | All Sections except 2.4, 5.2, 6.1.2, 9.3.2, and 9.4. |
| Win-Kuang Shen | Mayo Clinic Arizona—Professor of Medicine; Chair, Division of Cardiovascular Diseases | None | None | None | None | None | None | None |
| Cynthia M. Tracy | George Washington University—Professor of Medicine; Associate Director Division of Cardiology, Director of Cardiac Services | None | None | None | None | None | None | None |
| Reviewer | Representation | Employment | Consultant | Speakers Bureau | Ownership/ Partnership/ Principal | Personal Research | Institutional, Organizational, or Other Financial Benefit | Expert Witness |
|---|---|---|---|---|---|---|---|---|
| Eugene H. Chung | Official Reviewer—HRS | University of North Carolina School of Medicine—Associate Professor of Medicine | None | None | None | None | • Zoll Medical† | None |
| Timm L. Dickfeld | Official Reviewer—HRS | University of Maryland School of Medicine—Associate Professor of Medicine; Baltimore Veterans Affairs Medical Center—Director, Electrophysiology | • Biosense Webster | None | None | • Biosense Webster*• General Electric* | None | None |
| Samuel S. Gidding | Official Reviewer—ACC/AHA Task Force on Clinical Practice Guidelines | Nemours Cardiac Center—Division Chief of Cardiology; Jefferson Medical College—Professor of Pediatrics | None | None | None | None | None | None |
| Richard J. Kovacs | Official Reviewer—ACC Board of Trustees | Krannert Institute of Cardiology—Professor of Clinical Medicine | • Biomedical Systems* | None | None | • Siemens† | • AstraZeneca (DSMB)• MED Institute*• Eli Lilly (DSMB)*• Teva Pharmaceuticals | None |
| Byron K. Lee | Official Reviewer—AHA | University of California San Francisco—Professor of Medicine | • Biotronik• Boston Scientific• St. Jude Medical | None | None | • Zoll Medical* | • CarioNet* | • Defendant, Boehringer Ingelheim, 2013† |
| Gregory F. Michaud | Official Reviewer—AHA | Harvard Medical School—Assistant Professor | • Boston Scientific• Medtronic• St. Jude Medical | None | None | • Biosense Webster*• Boston Scientific*• St. Jude Medical* | None | None |
| Simone Musco | Official Reviewer—ACC Board of Governors | The International Heart Institute of Montana Foundation—Cardiology Research Investigator | None | • Bristol-Myers Squibb• Sanofi-aventis | None | None | None | None |
| Mohan N. Viswanathan | Official Reviewer—AHA | University of Washington School of Medicine—Assistant Professor of Medicine | • Biosense Webster• Siemens†• St. Jude Medical | None | None | • Medtronic* | None | None |
| Seshadri Balaji | Content Reviewer | Oregon Health and Science University—Professor of Pediatrics and Pediatric Cardiology, Director of Pacing and Electrophysiology | None | None | None | • Medtronic* | None | None |
| Nancy C. Berg | Content Reviewer—ACC Electrophysiology Section | Allina Health System | None | None | None | None | None | None |
| Noel G. Boyle | Content Reviewer—ACC Electrophysiology Section | University of California Los Angeles—Clinical Professor of Medicine | None | None | None | None | None | None |
| A. John Camm | Content Reviewer | St. George's University of London—Professor of Clinical Cardiology | • Bayer*• Biotronik• Boehringer Ingelheim• Boston Scientific• ChanRx• Daiichi-Sankyo• Medtronic• Menarini• Mitsubishi• Novartis†• Richmond Pharmacology*• Sanofi-aventis• Servier Pharmaceuticals*• St. Jude Medical• Takeda Pharmaceuticals• Xention | • Pfizer | None | None | None | None |
| Robert M. Campbell | Content Reviewer—ACC Adult Congenital and Pediatric Cardiology Section | Sibley Heart Center Cardiology—Director, Chief of Cardiac Services; Emory University School of Medicine—Division Director of Pediatric Cardiology, Professor of Pediatrics | None | None | None | None | None | None |
| Susan P. Etheridge | Content Reviewer—ACC Adult Congenital and Pediatric Cardiology Section | University of Utah—Training Program Director | None | None | None | None | None | None |
| Paul A. Friedman | Content Reviewer | Mayo Clinic—Professor of Medicine; Cardiovascular Implantable Device Laboratory—Director | • NeoChord | None | None | • Biotronik†• Medtronic• St. Jude Medical | • Preventice• Sorin* | None |
| Bulent Gorenek | Content Reviewer—ACC Electrophysiology Section | Eskisehir Osmangazi University—Professor and Vice Director, Cardiology Department | None | None | None | None | None | None |
| Jonathan L. Halperin | Content Reviewer—ACC/AHA Task Force on Clinical Practice Guidelines | Mt. Sinai Medical—Professor of Medicine | • AstraZeneca• Bayer Healthcare• Biotronik†• Boehringer Ingelheim†• Boston Scientific• Daiichi-Sankyo• Johnson & Johnson• Medtronic• Pfizer | None | None | None | None | None |
| Warren M. Jackman | Content Reviewer | University of Oklahoma Health Sciences Center—George Lynn Cross Research Professor Emeritus; Heart Rhythm Institute—Senior Scientific Advisor | • Biosense Webster*• Boston Scientific*• VytronUS* | • AtriCure*• Biosense Webster*• Biotronik*• Boston Scientific* | None | None | None | None |
| G. Neal Kay | Content Reviewer | University of Alabama—Professor Emeritus | None | None | None | None | None | None |
| George J. Klein | Content Reviewer | London Health Sciences Center—Chief of Cardiology | • Biotronik• Boston Scientific• Medtronic† | None | None | None | None | None |
| Bradley P. Knight | Content Reviewer | Northwestern University—Professor of Cardiology | • Boston Scientific• Medtronic | • Biosense Webster• Biotronik• Boston Scientific• Medtronic | None | None | None | None |
| John D. Kugler | Content Reviewer | University of Nebraska Medical Center—Division Chief of Pediatric Cardiology | None | None | None | None | None | None |
| Fred M. Kusumoto | Content Reviewer | Mayo Clinic—Professor of Medicine | None | None | None | None | None | None |
| Glenn N. Levine | Content Reviewer—ACC/AHA Task Force on Clinical Practice Guidelines | Baylor College of Medicine—Professor of Medicine; Director, Cardiac Care Unit | None | None | None | None | None | None |
| Marco A. Mercader | Content Reviewer | George Washington University—Associate Professor of Medicine | None | None | None | None | None | None |
| William M. Miles | Content Reviewer | University of Florida—Professor of Medicine, Silverstein Chair for Cardiovascular Education, Director of the Clinical Cardiac Electrophysiology Fellowship Program | None | None | None | None | • Medtronic (DSMB) | None |
| Fred Morady | Content Reviewer | University of Michigan—McKay Professor of Cardiovascular Disease | None | None | None | None | None | None |
| Melvin M. Scheinman | Content Reviewer | University of California San Francisco—Professor of Medicine | • Amgen• Biosense Webster• Biotronik*• Boston Scientific*• Gilead Sciences• Janssen Pharmaceuticals• Medtronic• St. Jude Medical | None | None | None | None | None |
| Sarah A. Spinler | Content Reviewer | University of the Sciences, Philadelphia College of Pharmacy—Professor of Clinical Pharmacy | • Portola Pharmaceuticals | None | None | None | None | None |
| William G. Stevenson | Content Reviewer | Brigham and Women's Hospital—Director, Clinical Cardiac Electrophysiology Program | • St. Jude Medical | None | None | None | None | None |
| Albert L. Waldo | Content Reviewer | University Hospitals—Associate Chief of Cardiovascular Medicine for Academic Affairs; Case Western Reserve University School of Medicine—Professor of Medicine | • AtriCure• Biosense Webster*• CardioInsight• ChanRx• Daiichi-Sankyo• Gilead Sciences • Pfizer • St. Jude Medical* | • Bristol-Myers Squibb*• Janssen Pharmaceuticals• Pfizer* | None | • Gilead Sciences* | None | None |
| Edward Walsh | Content Reviewer | Harvard Medical School—Professor of Pediatrics; Boston Children's Hospital—Chief, Division of Cardiac Electrophysiology | • Biosense Webster† | None | None | None | None | None |
| Richard C. Wu | Content Reviewer | University of Texas Southwestern Medical Center—Professor of Internal Medicine, Director of Cardiac Electrophysiology Lab | None | None | None | • Boehringer Ingelheim• Janssen Pharmaceutical• Medtronic | None | None |
| ACHD | adult congenital heart disease |
| AF | atrial fibrillation |
| AT | atrial tachycardia |
| AV | atrioventricular |
| AVNRT | atrioventricular nodal reentrant tachycardia |
| AVRT | atrioventricular reentrant tachycardia |
| BP | blood pressure |
| CTI | cavotricuspid isthmus |
| ECG | electrocardiogram/electrocardiographic |
| ERC | Evidence Review Committee |
| EP | electrophysiological |
| GWC | guideline writing committee |
| IST | inappropriate sinus tachycardia |
| MAT | multifocal atrial tachycardia |
| PJRT | permanent form of junctional reciprocating tachycardia |
| PSVT | paroxysmal supraventricular tachycardia |
| RCT | randomized controlled trial |
| SCD | sudden cardiac death |
| SVT | supraventricular tachycardia |
| VT | ventricular tachycardia |
| WPW | Wolff-Parkinson-White |
Posted by: hachigianroosevelt457.blogspot.com
Source: https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000000311
